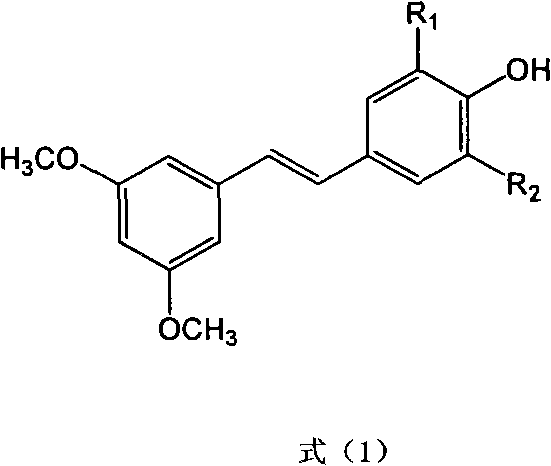
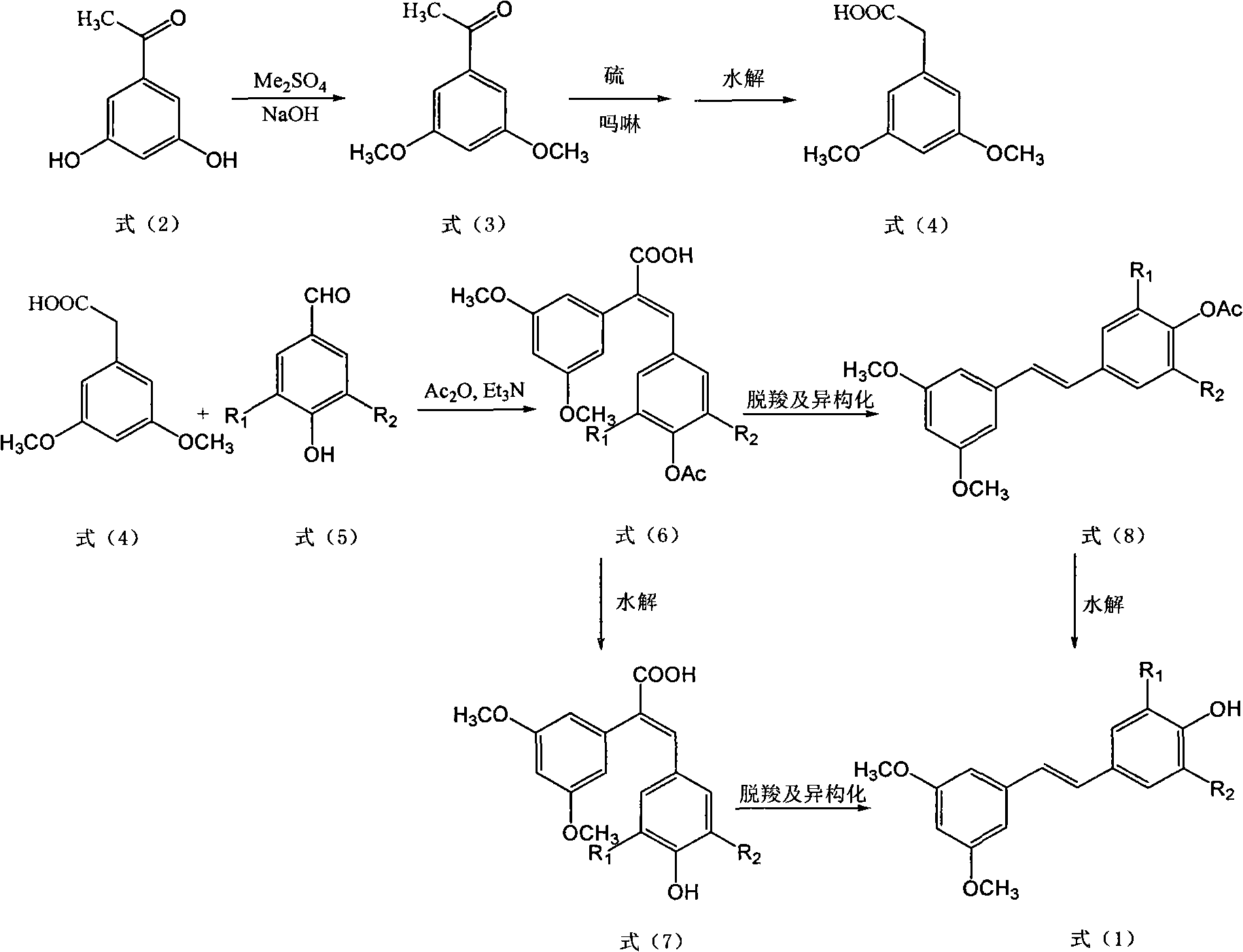
Methods for preparing E-3,5-dimethoxy-4'-oxhydryl diphenylethene and derivative thereof
A technology of hydroxystilbene and dimethoxyacetophenone, applied in the field of E-3, can solve the problems of low yield, cumbersome steps, difficult separation, etc., and achieves high selectivity, simple post-processing, and atom economy. Good results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] (1) Get 1.52g (10mmol) of 3,5-dihydroxyacetophenone, start dropwise addition of mass concentration simultaneously under stirring at room temperature and be 10%NaOH (20mmol) solution and Me 2 SO 4 2ml (20mmol), continue to stir for 2h after the dropwise addition, after the reaction is completed, pour the reaction solution into ice water and stir, a large amount of solids are precipitated, after standing, filter with suction, and the filter cake is air-dried to obtain 3,5-dimethoxybenzene Ethyl ketone 1.62g, yield 90%.
[0033] (2) Take 1.80g (10mmol) of 3,5-dimethoxyacetophenone, 0.64g (20mmol) of sulfur, 3ml (30mmol) of morpholine, and 0.05g (0.3mmol) of p-toluenesulfonic acid, at 120°C Stir for 8 hours, stop heating after the disappearance of the raw materials, add a mass concentration of 20% NaOH (50mmol) solution and 0.13g (0.04mmol) of tetrabutylammonium bromide to the reaction flask after the reaction liquid is cooled, and react at 100°C for 8 hours , then pour t...
Embodiment 2
[0039] (1) Get 1.52g (10mmol) of 3,5-dihydroxyacetophenone, start dropwise addition of mass concentration simultaneously under stirring at room temperature and be 18%NaOH (22mmol) solution and Me 2 SO 4 2.2ml (22mmol), continue to stir for 2h after the dropwise addition, after the reaction is completed, pour the reaction solution into ice water and stir, a large amount of solids are precipitated, after standing, filter with suction, and the filter cake is air-dried to obtain 3,5-dimethoxy Acetophenone 1.64g, yield 91%.
[0040] (2) Take 1.80g (10mmol) of 3,5-dimethoxyacetophenone, 0.64g (20mmol) of sulfur, 3ml (30mmol) of morpholine, and 0.06g (0.35mmol) of p-toluenesulfonic acid, at 120°C Stir for 8 hours, stop heating after the disappearance of the raw materials, add a mass concentration of 22% NaOH (50mmol) solution and 0.16g (0.05mmol) of tetrabutylammonium bromide to the reaction flask after the reaction liquid is cooled, and react at 100°C for 6 hours , then pour the r...
Embodiment 3
[0045] (1) Get 1.52g (10mmol) of 3,5-dihydroxyacetophenone, and the mass concentration is 20% NaOH (22mmol) aqueous solution, start to drop NaOH solution and Me 2 SO 4 2.2ml (22mmol), continue to stir for 3h after the dropwise addition, after the reaction is completed, pour the reaction solution into ice water and stir, a large amount of solids are precipitated, after standing, filter with suction, and the filter cake is air-dried to obtain 3,5-dimethoxy Acetophenone 1.66g, yield 92%.
[0046] (2) Take 1.80g (10mmol) of 3,5-dimethoxyacetophenone, 0.48g (15mmol) of sulfur, 3ml (30mmol) of morpholine, and 0.05g (0.3mmol) of p-toluenesulfonic acid, at 115°C Stir for 9 hours, stop heating after the disappearance of the raw materials, add a mass concentration of 28% NaOH (50 mmol) aqueous solution and 0.16 g (0.05 mmol) of tetrabutylammonium bromide to the reaction flask after the reaction liquid is cooled, and react at 100 ° C for 7 hours Pour the reaction solution into a beaker...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com