Preparation method of Naftopidil
A technology of naftopidil and naphthyloxy, which is applied in the field of preparation of naftopidil, can solve the problems of high energy consumption, poor color, low yield, etc., and achieve the effect of improving yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
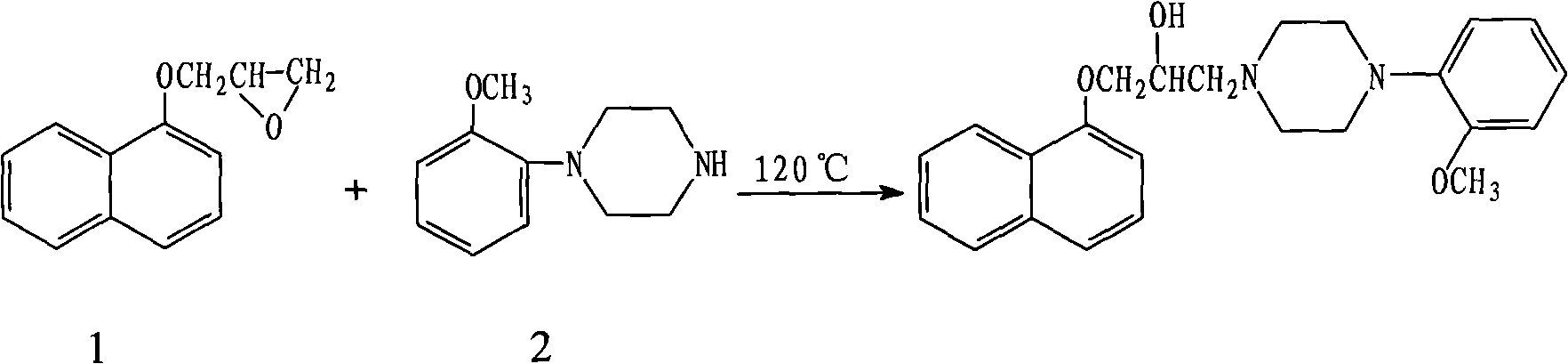
[0024] 1) Naftopidil preparation:
[0025] In a 5000ml three-necked bottle, install a thermometer, a condenser, and a mechanical stirrer. Add 3-(1-naphthyloxy)-1,2-propylene oxide 326.3g (content about 77%, 1.26mol), o-methoxyphenylpiperazine hydrobromide 366g (1.26mol), potassium carbonate 95.7 g (0.69 mol) was dissolved in 100 g of purified water and 600 ml of chloroform, and refluxed for 8 hours under stirring. Cool to room temperature, filter, and wash the filter residue with 300ml of chloroform. The filtrate was washed twice with purified water, the organic layer was separated, and concentrated to dryness under reduced pressure to obtain 551 g of a black oil. Add 700ml of absolute ethanol, heat to dissolve, add 15g of activated carbon, continue to heat and reflux for 10 minutes, suction filter while hot, cool to room temperature with stirring, cool in an ice-water bath for 3 hours, precipitate a large amount of crystals, filter with suction, and dry to obtain a yellowis...
Embodiment 2
[0029] In a 5000ml three-necked bottle, install a thermometer, a condenser, and a mechanical stirrer. Add 3-(1-naphthyloxy)-1,2-propylene oxide 300g (content about 77%, 1.15mol), o-methoxyphenylpiperazine hydrobromide 350g (1.20mol), potassium carbonate 90g (0.65mol) was dissolved in 100g of purified water, 600ml of chloroform, and refluxed under stirring for 7 hours. Cool to room temperature, filter, and wash the filter residue with 300ml of chloroform. The filtrate was washed twice with purified water, the organic layer was separated, and concentrated to dryness under reduced pressure to obtain 540 g of a black oil. Add 700ml of absolute ethanol, heat to dissolve, add 15g of activated carbon, continue to heat and reflux for 20 minutes, suction filter while hot, cool to room temperature with stirring, cool in an ice-water bath for 3 hours, precipitate a large amount of crystals, filter with suction, and dry to obtain a yellowish solid 492g, yield 83.6%. mp: 125-127°C, the ...
Embodiment 3
[0033] In a 5000ml three-necked bottle, install a thermometer, a condenser, and a mechanical stirrer. Add 245g of 3-(1-naphthyloxy)-1,2-propylene oxide (content about 77%, 0.94mol), o-methoxyphenylpiperazine hydrobromide 276g (0.95mol), potassium carbonate 76g (0.55mol) was dissolved in 80g of purified water and 480ml of dichloromethane, and refluxed under stirring for 6.0 hours. Cool to room temperature, filter, and wash the filter residue with 250ml of dichloromethane. The filtrate was washed twice with purified water, the organic layer was separated, and concentrated to dryness under reduced pressure to obtain 450 g of a black oil. Add 600ml of absolute ethanol, heat to dissolve, add 15g of activated carbon, continue to heat and reflux for 10 minutes, suction filter while it is hot, cool to room temperature while stirring, and cool in an ice-water bath for 5 hours, a large amount of crystals precipitate, suction filter, and dry to obtain a yellowish solid 402g, yield 83.7...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com