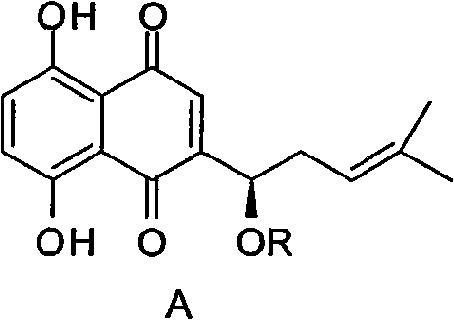
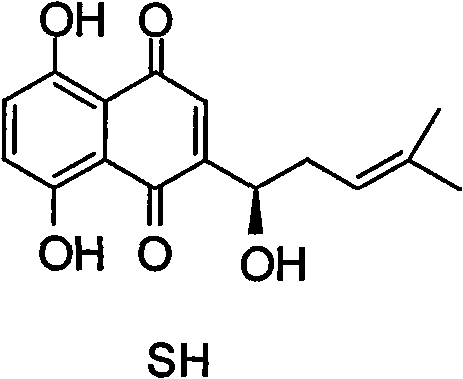
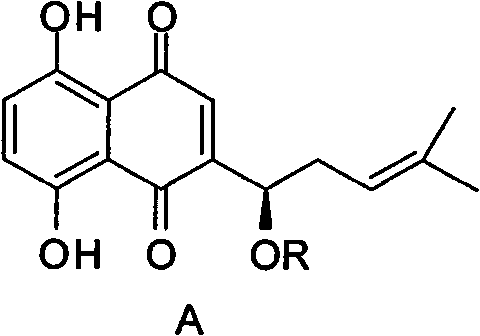
Alkannin acetyl glucose and preparation method and application thereof
A technology of shikonin and acetyl sugar, applied in the field of shikonin acetyl sugar and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] The reaction formula of the preparation method of the derivative of shikonin acetyl sugar is as follows:
[0031]
[0032] Aldose 1 (a~k) is acetylated under the action of acetic anhydride and sodium acetate, the acetic anhydride is 10 times the molar amount of 1, and the sodium acetate is the equimolar amount of 1, the reaction temperature is controlled at 80°C, and the reaction time is 4 hours , TLC tracking of the reaction process. After the reaction is completed, cool to room temperature, pour the reaction solution into an ice-cold saturated sodium bicarbonate solution to neutralize the remaining acetic anhydride, continue stirring for 1.5 hours, then add a suitable extractant for extraction, such as dichloromethane, etc., and purify by column chromatography. The peracetyl sugars 2(a~k) were obtained.
[0033] Peracetylsugar 2 (a~e, i~k) was then reacted with hydrazine acetate at room temperature for 4 hours, tetrahydrofuran was used as a solvent, and the amount...
Embodiment 1
[0043] 1-(1-(5,8-dihydroxy-1,4-naphthoquinone-2-yl)-4-methyl-3-pentyl)-2,3,4,6-tetra-O-acetyl -β-D-glucopyranose
[0044] Add 1.80g (10mmol) of glucose and 1.07g (13mmol) of anhydrous sodium acetate into a single-necked flask, add 9.4mL (100mmol) of acetic anhydride, control the reaction temperature at 80-90°C, and the reaction time is about 4 hours. TLC tracking. After the reaction is completed, cool to room temperature, pour the reaction solution into ice-cold saturated sodium bicarbonate solution, neutralize the remaining acetic anhydride, continue to stir for 1.5 hours, then add a suitable extractant for extraction, such as dichloromethane, etc., then separate and pass Purified by column chromatography to obtain 3.62 g (92.8% yield) of 1,2,3,4,6-penta-O-acetylglucose.
[0045] Dissolve 1.95 g (5 mmol) of pentaacetylglucose in 20 mL of tetrahydrofuran, add 0.506 g (5.5 mmol) of hydrazine acetate and stir at room temperature for 4 hours. After the reaction, tetrahydrofuran ...
Embodiment 2
[0051] 1-(1-(5,8-dihydroxy-1,4-naphthoquinone-2-yl)-4-methyl-3-pentyl)-2,3,4,6-tetra-O-acetyl -β-D-mannopyranose
[0052] The operation was the same as in Example 1, except that glucose was replaced by mannose to obtain the title compound with the following structure.
[0053] 1 H NMR (400MHz, CDCl 3 ): δ12.57(s, 1H), 12.46(s, 1H), 7.18(m, 3H), 5.30(m, 2H), 5.23(t, J=10Hz, 1H), 5.12(t, J=6.8 Hz, 1H), 5.04(m, 2H), 4.14(dd, J=5.6, 12.0Hz, 1H), 3.89(dd, J=1.6, 12.4Hz, 1H), 3.83(m, 1H), 2.51(m , 1H), 2.45(m, 1H), 2.14(s, 3H), 2.02(s, 3H), 2.00(s, 3H), 1.94(s, 3H), 1.67(s, 3H), 1.58(s, 3H) ppm;
[0054] 13 C NMR (100MHz, CDCl 3 ): δ177.9, 176.4, 170.4, 169.9, 169.7, 169.7, 167.8, 167.3, 148.9, 136.6, 133.1, 132.7, 132.1, 117.4, 111.7, 111.5, 97.5, 73.2, 69.4, 69.3, 68.9, 2 33.5, 25.7, 20.8, 20.7, 20.6, 20.4, 18.0ppm;
[0055]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com