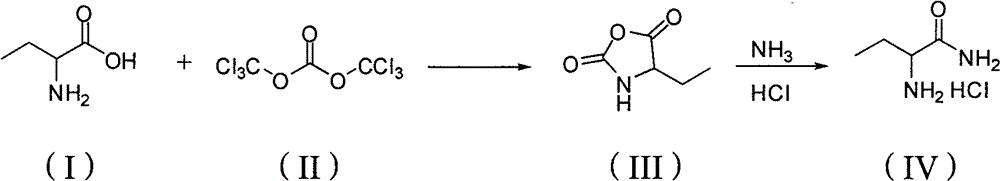Chemical synthesis method for of 2-amino-butanamide hydrochloride
An aminobutyramide, chemical synthesis technology, applied in chemical instruments and methods, organic chemistry, preparation of carboxylic acid amides, etc., can solve the problems of large environmental pollution of sulfur-containing wastewater, poor absorption effect of sulfur dioxide, etc., and achieve great practical value and social Economic benefits, low production cost and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
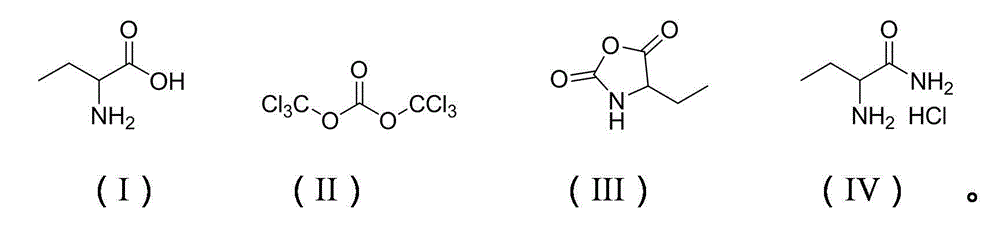
[0022] The ratio of the amount of feed material to 2-aminobutyric acid: bis(trichloromethyl)carbonate: organic amine is 1: 0.8: 0.2, using tetrahydrofuran as a solvent, and the quality of the solvent is 4 times that of 2-aminobutyric acid, and the organic amine For 1,3-dimethyl-2-imidazolidinone.
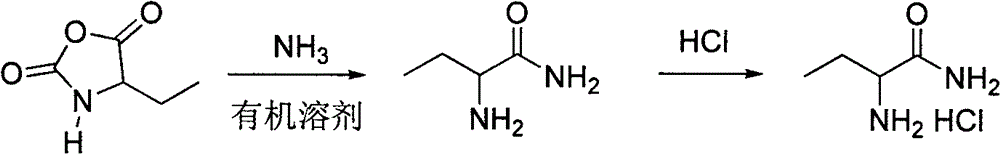
[0023] Dissolve 23.76g (80mmol) of bis(trichloromethyl)carbonate in 41g of tetrahydrofuran, and put it into a 250mL constant pressure dropping funnel. In a 250mL three-necked flask equipped with a thermometer, reflux condenser absorption device and mechanical stirring, add 10.3g (100mmol) of 2-aminobutyric acid and 2.28g of 3-dimethyl-2-imidazolidinone ( 20mmol). A solution of bis(trichloromethyl)carbonate was added dropwise to the flask at reflux temperature and reacted for 8 hours. Cooled to room temperature, distilled to obtain a pink oil and recrystallized from petroleum ether to obtain a white solid, namely 11.9 g of 4-ethyl-2,5-oxazolidinedione, with a yield of 92.1% and a p...
Embodiment 2
[0026] The ratio of the amount of feed material to 2-aminobutyric acid: bis(trichloromethyl)carbonate: organic amine is 1: 1.2: 0.01, and ethyl acetate is used as the organic solvent, and its consumption is 20 times the quality of 2-aminobutyric acid , the organic amine is triethylamine.
[0027] The reaction temperature was reflux temperature, and the reaction time was 10 hours. Other operations were the same as in Example 1 to obtain 11.3 g of 4-ethyl-2,5-oxazolidinedione with a yield of 87.6% and a purity of 98.1%.
Embodiment 3
[0029] The ratio of the amount of feed material to 2-aminobutyric acid: bis(trichloromethyl)carbonate: organic amine is 1: 1.5: 0.4, and ethyl acetate is used as the organic solvent, and its consumption is 30 times the quality of 2-aminobutyric acid , the organic amine is pyridine.
[0030] The reaction temperature was reflux temperature, and the reaction time was 8 hours. Other operations were the same as in Example 1 to obtain 11.0 g of 4-ethyl-2,5-oxazolidinedione with a yield of 85.3% and a purity of 98.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com