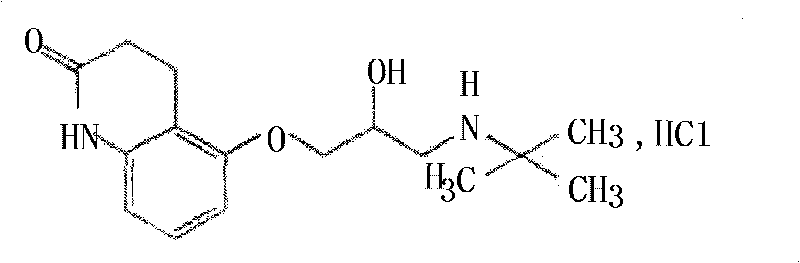
Carteolol orally disintegrating tablets and preparation method thereof
A technology of orally disintegrating tablets and hydrochloric acid cards, which is applied in the field of pharmaceutical preparations, can solve the problems that have not yet been reported on carteolol hydrochloride orally disintegrating tablets, and achieve the effects of good medication compliance, convenient taking, and broad market prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
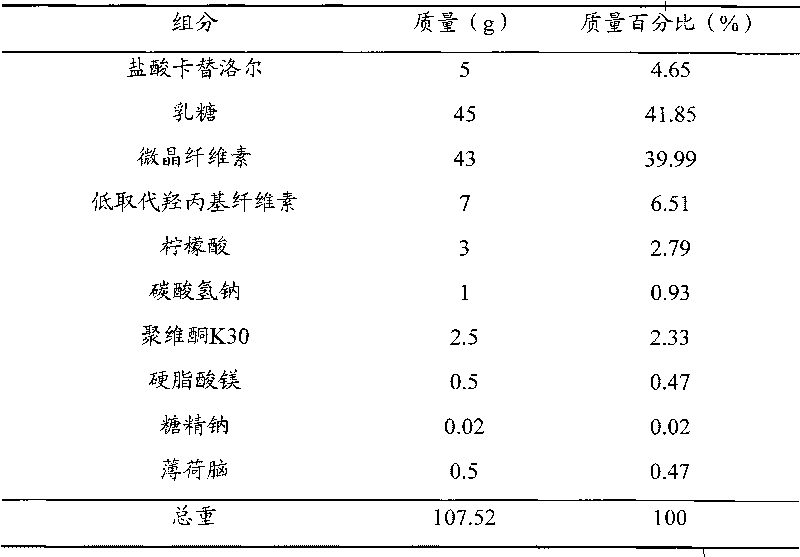
[0046] prescription
[0047]
[0048] manufacturing method Pulverize all the components in the prescription into fine powder with a fineness of more than 100 meshes; 5g carteolol hydrochloride, 45g lactose, 21.5g microcrystalline cellulose, 3.5g low-substituted hydroxypropyl cellulose, 3g citric acid , 0.02 g of sodium saccharin and 0.5 g of menthol were mixed, and 4% povidone K30 solution was added to make a soft material, granulated with a 24-mesh sieve, dried at 50 °C, and the dry granules were sieved through a 24-mesh sieve to granulate; Add 21.5 g of microcrystalline cellulose, 3.5 g of low-substituted hydroxypropyl cellulose, 1 g of sodium bicarbonate and 0.5 g of magnesium stearate to the dry granules, mix well, and press into tablets to make carteolol hydrochloride orally disintegrating. Dissolve 1000 tablets, each tablet contains carteolol hydrochloride 5mg.
[0049] quality The obtained orally disintegrating tablet has a smooth and glossy appearance; it ...
Embodiment 2
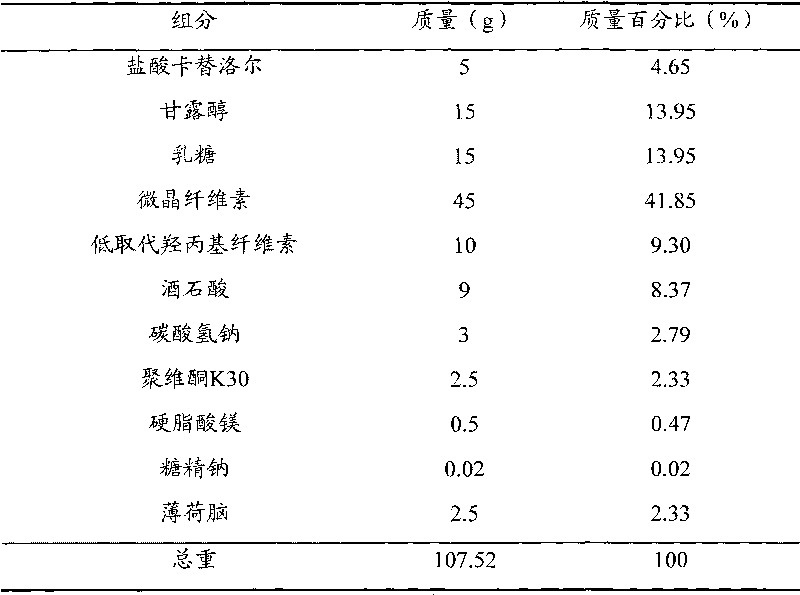
[0051] prescription
[0052]
[0053] manufacturing method Pulverize all the components in the prescription into fine powder with a fineness of more than 100 meshes; 5 g of carteolol hydrochloride, 15 g of mannitol, 15 g of lactose, 22.5 g of microcrystalline cellulose, 5 g of low-substituted hydroxypropyl cellulose, 9 g of tartaric acid, 0.02 g of sodium saccharin and 2.5 g of menthol were mixed, and 4% povidone K30 solution was added to make a soft material, granulated with a 24-mesh sieve, dried at 50°C, and the dried granules passed through a 24-mesh sieve to granulate ; Add 22.5 g of microcrystalline cellulose, 5 g of low-substituted hydroxypropyl cellulose, 3 g of sodium bicarbonate and 0.5 g of magnesium stearate to the dry granules, mix well and press into tablets to make carteolol hydrochloride oral cavity 1000 disintegrating tablets, each containing carteolol hydrochloride 5mg.
[0054] quality The obtained orally disintegrating tablet has a smooth and g...
Embodiment 3
[0056] prescription
[0057]
[0058] manufacturing method All components in the prescription were pulverized into fine powder with a fineness of more than 100 meshes; carteolol hydrochloride 5g, mannitol 57g, lactose 16g, microcrystalline cellulose 5.5g, low-substituted hydroxypropyl cellulose 1g, 7g of citric acid, 0.02g of sodium saccharin and 2.5g of menthol were mixed, and 4% povidone K30 solution was added to make a soft material, granulated with a 24-mesh sieve, dried at 50°C, and the dry granules were sieved through a 24-mesh sieve. Then add 5.5g of microcrystalline cellulose, 1g of low-substituted hydroxypropyl cellulose, 4g of sodium bicarbonate and 0.6g of magnesium stearate to the dry granules, mix well and press into tablets to make carteolol hydrochloride. 1000 orally disintegrating tablets, each containing carteolol hydrochloride 5mg.
[0059] quality The obtained orally disintegrating tablet has a smooth and glossy appearance; completely disintegra...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Fineness | aaaaa | aaaaa |
| Fineness | aaaaa | aaaaa |
| Fineness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com