Polarimetric polyurethane-urea infrared low emissivity material and preparation method thereof
A technology of polyurethane urea and low emissivity, applied in the field of optically active polyurethane urea infrared low emissivity materials and its preparation, can solve the problems of low emissivity indium tin oxide powder, etc., and achieve superior processing performance, conformational stability and thermal stability Good, highly optically active results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
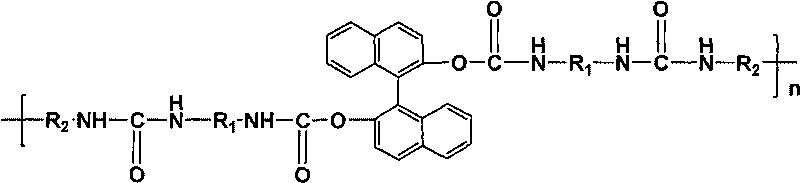
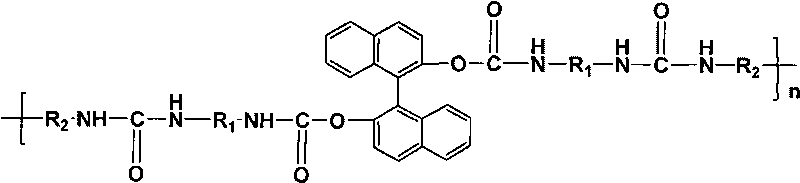
[0022] N 2 Under atmosphere, heat 20mL N,N-dimethylformamide (DMF) to 75-80°C, add R-type 1,1'-binaphthyl-2,2'-diphenol (R -BINOL) 0.572g, continue to heat up to 100°C after dissolving it. Then, 20 mL of DMF solution of 0.2 mol / L toluene-2,4-diisocyanate (TDI) was slowly added dropwise, and reacted for 6 h to obtain the polyurethane urea prepolymer terminated by isocyanate group; N 2 Under atmosphere, naturally cool the above polyurethaneurea prepolymer to 75°C, add m-phenylenediamine (m-PhDA) 0.216g dissolved in 10mL DMF, react at 75°C for 5h, distill under reduced pressure to remove the DMF solvent, 45°C Under vacuum drying for 12 hours, the crude product of optically active polyurethane urea was obtained; the crude product of optically active polyurethane urea was washed 3 to 5 times with 150mL of absolute ethanol, and after vacuum drying at 30°C for 24 hours, an optically active polyurethane urea material with low infrared emissivity was obtained. The optical rotation of ...
Embodiment 2
[0029] N 2 Under atmosphere, heat 20mL N,N-dimethylformamide (DMF) to 75-80°C, add S-type 1,1'-binaphthyl-2,2'-diphenol (S- BINOL) 0.572g, continue to heat up to 100°C after dissolving it. Then, 20 mL of DMF solution of 0.2 mol / L toluene-2,4-diisocyanate (TDI) was slowly added dropwise, and reacted for 6 h to obtain the polyurethane urea prepolymer terminated by isocyanate group; N 2 Under the atmosphere, the above-mentioned polyurethane urea prepolymer was naturally cooled to 75 ° C, and 0.454 g of 4,4′-diaminobenzanilide dissolved in 10 mL of DMF was added, reacted at 75 ° C for 5 h, and the DMF solvent was removed by distillation under reduced pressure. Vacuum-dried at 45°C for 12 hours to obtain the crude product of optically active polyurethane urea; washed the crude product of optically active polyurethane urea with 250mL of absolute ethanol for 3 to 5 times, and vacuum-dried at 30°C for 12 hours to obtain the material of optically active polyurethane urea with low infr...
Embodiment 3
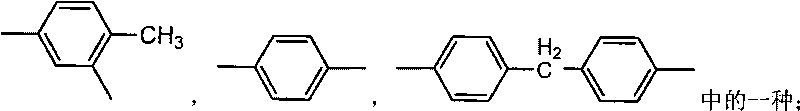
[0036] N 2 Under atmosphere, heat 20mL N,N-dimethylformamide (DMF) to 80°C, add S-type 1,1'-binaphthyl-2,2'-diol (S-BINOL) with an optical purity of 90% 0.572g, after dissolving it, continue to heat up to 100°C. Then, slowly drop 1g 4,4'-diphenylmethane diisocyanate (MDI) that is dissolved in 20mL DMF, react 6h, make the polyurethane urea prepolymer of isocyanate group termination; N 2 Under atmosphere, naturally cool the above polyurethaneurea prepolymer to 75°C, add m-phenylenediamine (m-PhDA) 0.216g dissolved in 10mL DMF, react at 75°C for 5h, distill under reduced pressure to remove the DMF solvent, 45°C Under vacuum drying for 12 hours, the crude product of optically active polyurethane urea was obtained; the crude product of optically active polyurethane urea was washed 3 to 5 times with 300 mL of absolute ethanol, and after vacuum drying at 30°C for 12 hours, an optically active polyurethane urea with low infrared emissivity material was obtained. The optical rotation ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thermal decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com