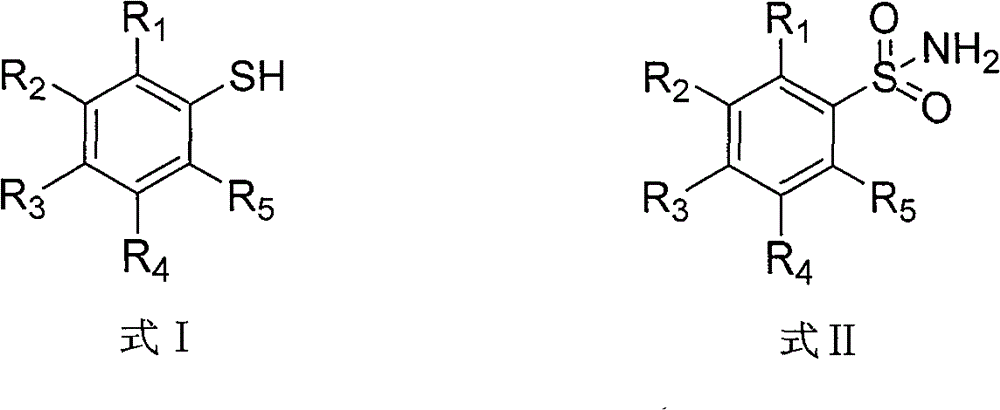Preparation method of thiophenol
A technology of thiophenols and compounds, which is applied in the field of preparation of thiophenols, can solve problems such as environmental pollution, increase production costs, and increase separation costs, and achieve the effects of reducing production costs, simplifying separation processes, and high chemical selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0013] Embodiment 1: the preparation of 4-methylthiophenol
[0014] Mix 8.5g of 4-methylbenzenesulfonamide (about 0.05mol) with 12.0g of ammonium formate (about 0.19mol), heat to 180°C for 5 hours, and distill off the water generated during the reaction. After the reaction, the temperature of the reaction liquid was lowered to 80° C., and the pH of the reaction liquid was adjusted to 2 by adding dilute hydrochloric acid with a mass concentration of 10%. Then rectification was performed to obtain 4.5 g of 4-methylthiophenol.
[0015] The conversion rate of 4-methylbenzenesulfonamide was 73.2%, and the yield of 4-methylthiophenol was 71.8%.
Embodiment 2
[0016] Embodiment 2: the preparation of 4-hydroxybenzenethiol
[0017] Mix 8.7g of 4-hydroxybenzenesulfonamide (about 0.05mol) with 15.0g of ammonium formate (about 0.24mol), heat to 200°C for 4 hours, and distill the water generated during the reaction. After the reaction, the temperature of the reaction solution was lowered to 80° C., and 10% dilute sulfuric acid was added to adjust the pH to 2, followed by rectification to obtain 4.7 g of 4-hydroxythiophenol.
[0018] The conversion rate of 4-hydroxybenzenesulfonamide was 75.5%, and the yield of 4-hydroxybenzenethiophenol was 74.1%.
Embodiment 3
[0019] Embodiment 3: Preparation of 4-fluorothiophenol
[0020] Mix 8.7g of 4-fluorobenzenesulfonamide (about 0.05mol) with 15.0g of potassium formate (about 0.18mol), heat to 210°C for 6 hours, and distill off the water generated during the reaction. After the reaction, the temperature of the reaction liquid was lowered to 80° C., and 10% dilute sulfuric acid was added to adjust the pH to 2, followed by rectification to obtain 5.3 g of 4-fluorothiophenol.
[0021] The conversion rate of 4-fluorobenzenesulfonamide was 84.3%, and the yield of 4-fluorobenzenethiol was 82.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com