Water soluble puerarin derivatives and preparation method and application thereof
A technology for puerarin derivatives and puerarin, which is applied in the directions of sugar derivatives, chemical instruments and methods, and compounds of elements of Group 5/15 of the periodic table, etc., can solve the problems of unstable preparations and poor water solubility of puerarin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
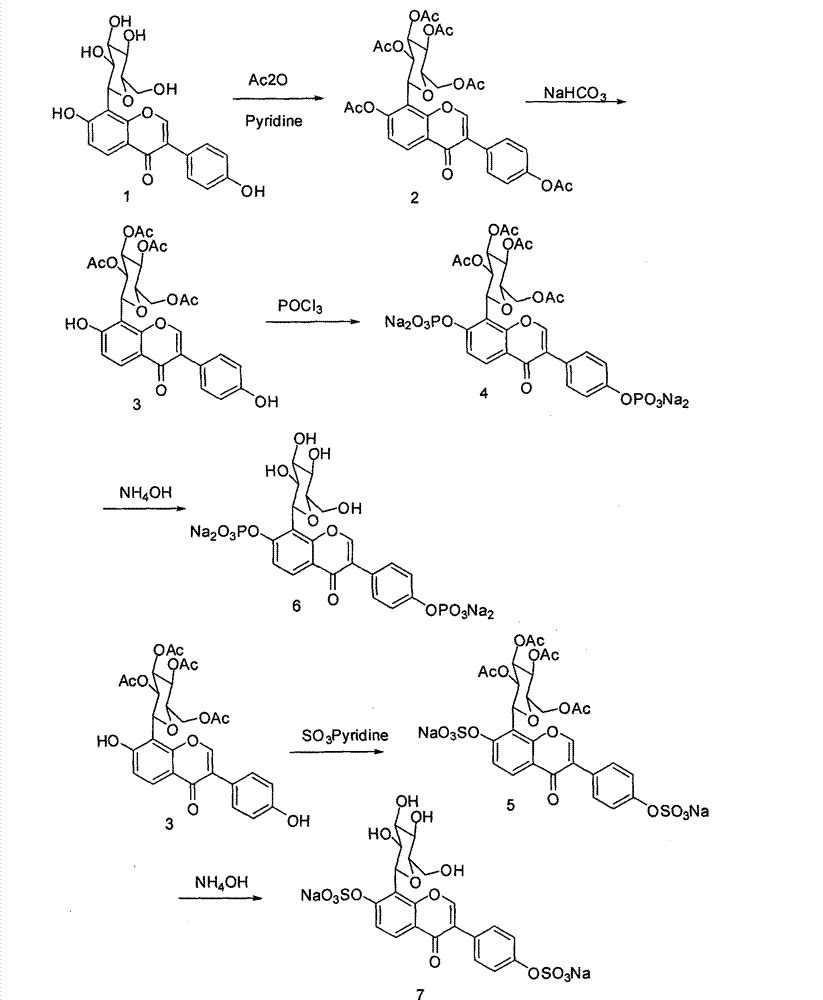
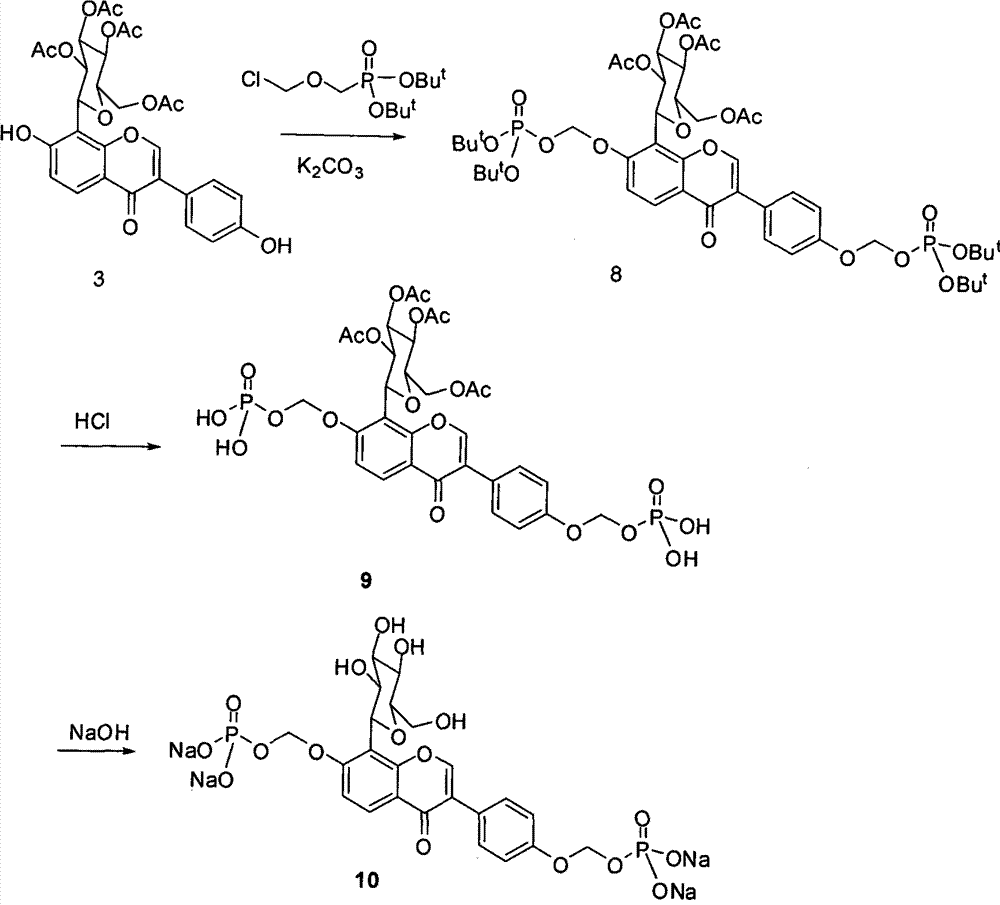
[0056] Dissolve 4.5 g (10.8 mmol) of puerarin (expressed in Formula 1) in 30 mL of dry pyridine, and slowly add 7.5 mL of acetic anhydride with stirring at room temperature. Stirring was continued at room temperature for 24 hours. Pyridine was evaporated under reduced pressure, and dilute HCl was added to adjust the pH to about 6. Use ethyl acetate, continue to stir for 24 hours, TLC tracking until the raw material point disappears (developing agent dichloromethane: methanol 9:1), evaporate pyridine under reduced pressure, add dilute HCl aqueous solution to adjust the pH value to 6, and extract with ethyl acetate , MgSO 4 After drying, filter, and evaporate to dryness of ethyl acetate to obtain a crude product, which was separated by column chromatography, the eluent was dichloromethane: methanol = 25:1, collected and evaporated to dryness to obtain 5.05 g of white powder acetyl puerarin (shown in formula 2). H1 NMR (CDCl3): δ8.20(d, J=8.0Hz, 1H), 8.00(s, 1H), 7.57(d, J=8.4H...
Embodiment 2
[0058] At room temperature, dissolve 5 g (7.48 mmol) of white powder acetyl puerarin (expressed in formula 2) in 100 mL of dry dichloromethane, slowly add 30 mL of saturated sodium bicarbonate solution and stir, TLC traces to the raw material Point disappears, adding appropriate amount of water, dichloromethane extraction, anhydrous Na 2 SO 4 After drying, it was filtered, and the filtrate was evaporated to dryness to obtain a crude product, which was purified by column chromatography with the elution condition of dichloromethane:methanol=45:1 to obtain 3.2 g of a white solid (shown in Formula 3), with a yield of 69.4%. H1NMR (CDCl3): δ8.30(br s, 1H), 8.21(d, J=8.8HZ, 1H), 7.99(s, 1H), 7.39(d, J=7.6HZ, 2H), 7.28(s, 1H), 7.03(d, J=8.8HZ, 1H), 6.89(d, J=7.6HZ, 2H), 5.99(br s, 1H), 5.48(m, 3H), 5.37(m, 1H), 4.40 (dd, J=8.0, 3.6HZ, 1H), 4.25(d, J=8.0Hz, 1H), 4.00(m, 1H), 2.16(s, 3H, 2, 12(s, 3H), 2.06(s , 3H), 1.71 (s, 3H).
Embodiment 3
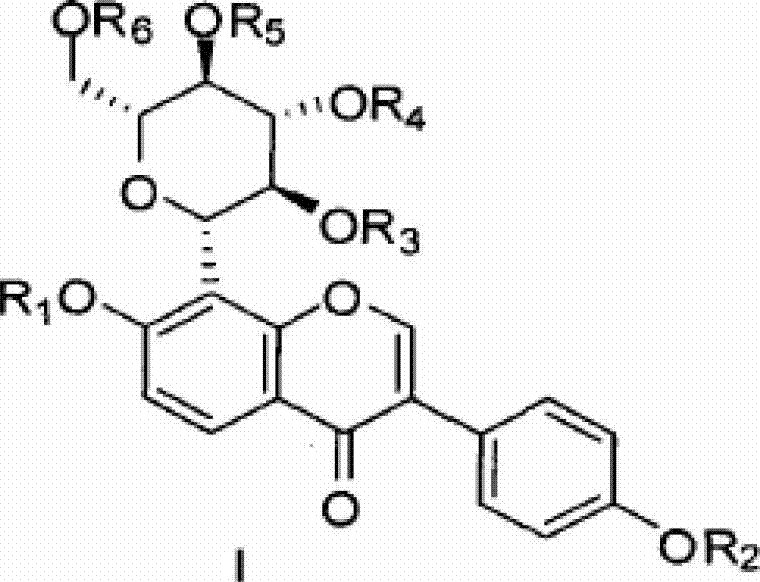
[0060] Dissolve 1 gram of tetraacetylpuerarin (compound shown in formula 3) in dry pyridine, stir at room temperature, add 1.57 grams of phosphorus oxychloride (POCl 3 ), stirred for 24 hours after the addition; thin-layer detection tracked until the raw material point disappeared (the developing agent was dichloromethane:methanol==13:1, v / v), evaporated the pyridine, added an appropriate amount of water, and used 2N NaOH as the eluent The solution was neutralized to pH 7, the macroporous resin AB-8 was separated, eluted with pure water and collected, and the water was evaporated to dryness under reduced pressure to obtain 500 mg of tetrahydroxypuerarin phosphate white powder (shown in formula 4).
[0061] At room temperature, its solubility in water is greater than 200mg / 100ml. A certain amount of sample was mixed with rat anticoagulant plasma, incubated at 37°C, and the drug was extracted with acetonitrile at different time points for HPLC analysis. The half-life of puerarin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com