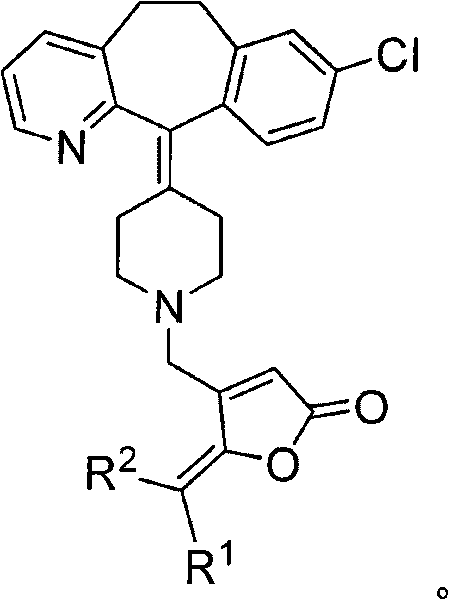
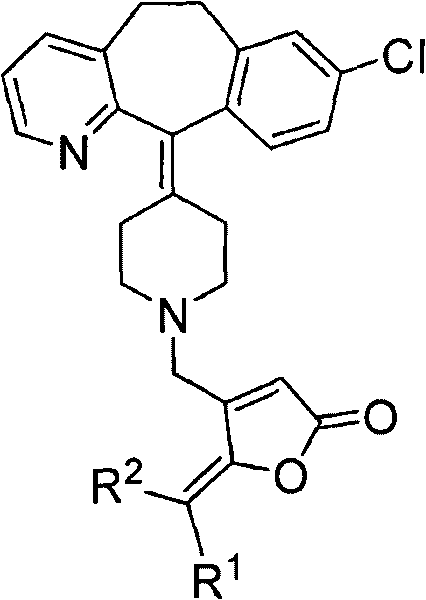
Desloratadine derivative containing gamma-subunit butenolide and synthesizing method thereof
A technology of subunit butenolactone and desloratadine, which is applied in the field of novel desloratadine derivatives and their synthesis, can solve the problems of unseen desloratadine derivatives, and achieve simple and convenient preparation methods with good inhibition role, the effect of broadening the structure type
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
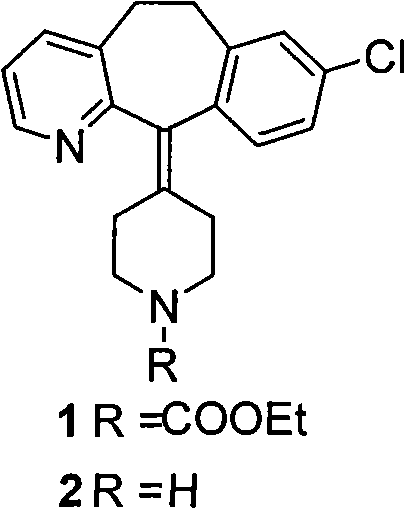
[0024] The preparation of desloratadine (2): loratadine (1) is placed in 80% ethanol solvent, the potassium hydroxide of 15 times of molar weight is catalyst, nitrogen protection, reflux 8-12 hour until reaction is complete, pump out The ethanol solvent was distilled off, extracted with saturated saline and water, and recrystallized with ethyl acetate to obtain white crystals to obtain desloratadine (2).
[0025] The preparation of compound 6: add 31g desloratadine (2) and 211g of 4-bromomethyl-furan-2-5H-ketone to carbon tetrachloride solution, triethylamine of catalytic amount, stir at room temperature for 6 hours, TLC The reaction was monitored for completion. The reaction mixture was extracted with ethyl acetate, the reaction system was concentrated, and separated by column chromatography to obtain 26 g of pink oil with a yield of 65%, namely compound 6. The experimental data are as follows:
[0026] IR (KBr, cm -1 ): 1776, 1748, 1637, 1589, 1476, 1438, 1142, 1031, 886,...
Embodiment 1
[0027] Embodiment 1: R shown in the preparation general formula II 1 = o-methoxyphenyl, R 2 = Derivatives of H (II-1)
[0028] 406 mg of compound 6 and 204 mg of o-methoxybenzaldehyde were dissolved in 15 mL of methanol, 50 mg of sodium carbonate was added, and reacted at room temperature for 6 hours. After the reaction, add 15 mL of chloroform to dilute, add silica gel to the suction filter funnel and filter to remove the alkali in the system, concentrate the reaction system, and separate by column chromatography to obtain 210 mg of a light yellow solid, which is a single Z-isomer, with a yield of 40%. . The experimental data are as follows:
[0029] m.p=117-119°C.IR(KBr, cm -1 ): 1766, 1598, 1488, 1464, 1438, 1247, 1112, 1024, 826, 756. 1 H NMR (400MHz, CDCl 3, TMS): δ8.41(d, J=4.2Hz, 1H), 8.21(d, J=7.6Hz, 1H), 7.46(d, J=7.4Hz, 1H), 7.31-7.28(m, 1H) , 7.17-7.09(m, 4H), 7.01(m, 1H), 6.92(s, 1H, =C (7″) H), 6.91-6.88(m, 1H), 6.14(s, 1H, =C (3″) H), 3.90(s, 3H, OCH 3 ...
Embodiment 2
[0030] Embodiment 2: R shown in the preparation general formula II 1 = p-Hydroxyphenyl, R 2 = Derivatives of H (II-2)
[0031] Dissolve 406mg of compound 6 and 366mg of p-hydroxybenzaldehyde in 15mL of ethanol, add 50mg of potassium carbonate, and react at room temperature for 24 hours. After the reaction, add 20 mL of chloroform to dilute, add silica gel to the suction filter funnel and filter to remove the alkali in the system, concentrate the reaction system, ethyl acetate crystallizes to obtain 459 mg of a light yellow solid, which is a single Z-isomer, and the yield is 90%. . The experimental data are as follows:
[0032] m.p=198-200℃.IR(KBr, cm -1 ): 1747, 1605, 1573, 1513, 1439, 1371, 1290, 1222, 1166, 928, 827. 1 H NMR (400MHz, CDCl 3 , TMS): δ8.39(d, J=4.6Hz, 1H), 7.66(d, J=8.6Hz, 2H), 7.51(d, J=7.5Hz, 1H), 7.17-7.05(m, 4H) , 6.89(d, J=8.6Hz, 2H), 6.42(s, 1H, =C (7″) H), 6.03(s, 1H, =C (3″) H), 3.48(s, 2H), 3.41-3.37(m, 2H), 2.84-2.76(m, 4H), 2.41-2.38(m, 2H)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com