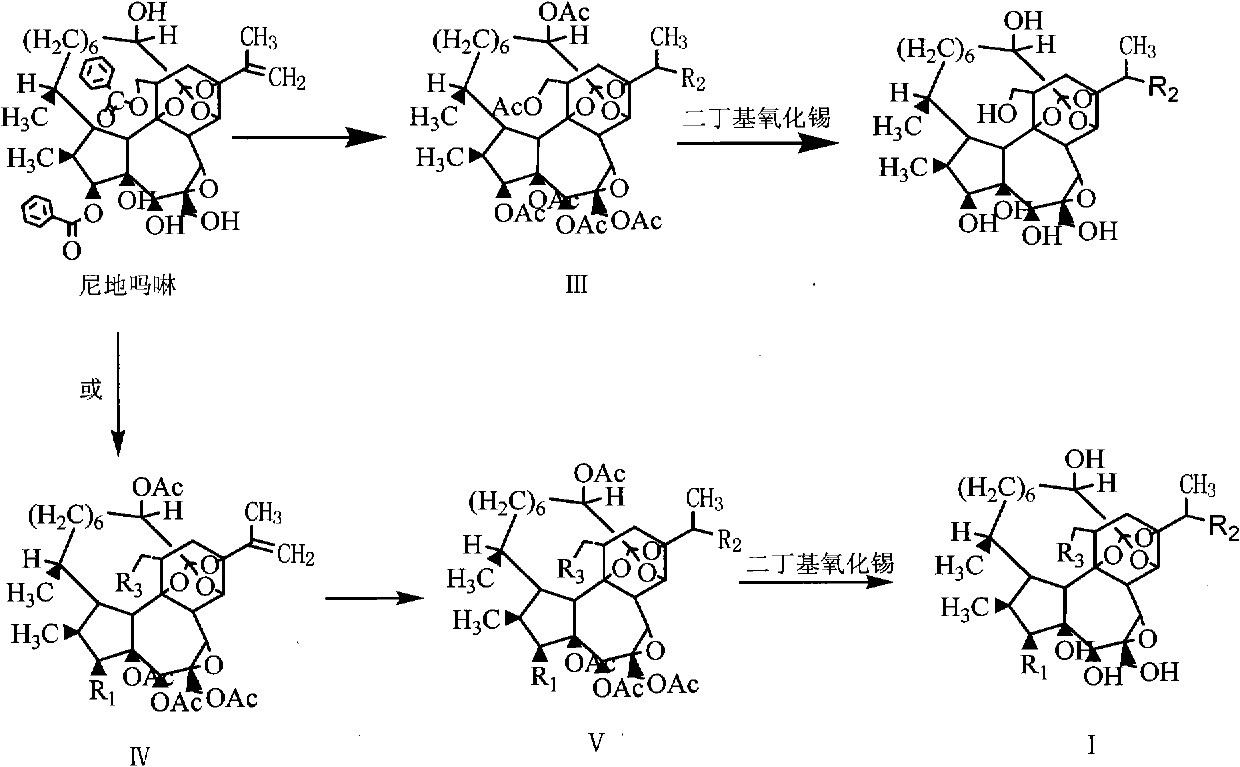
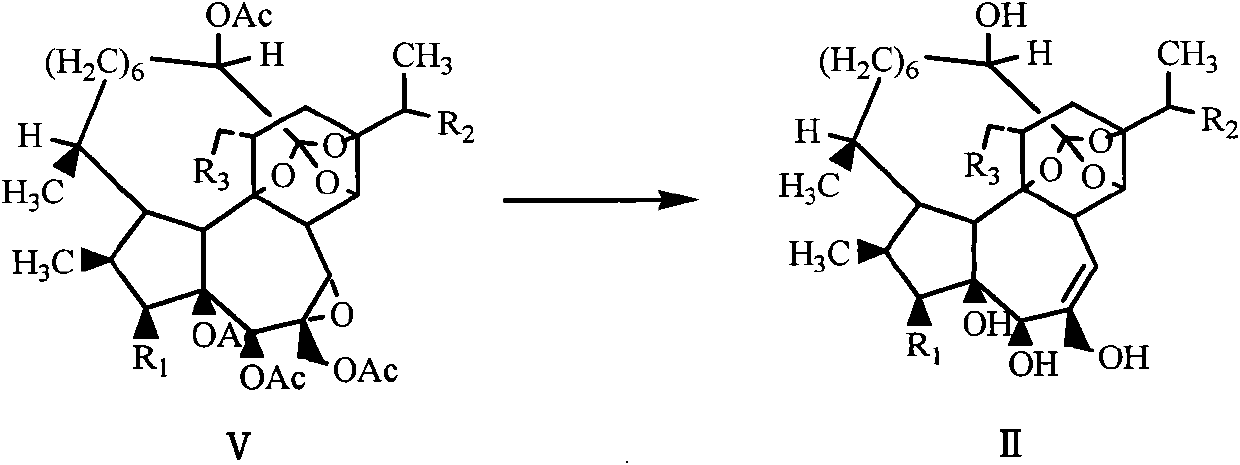
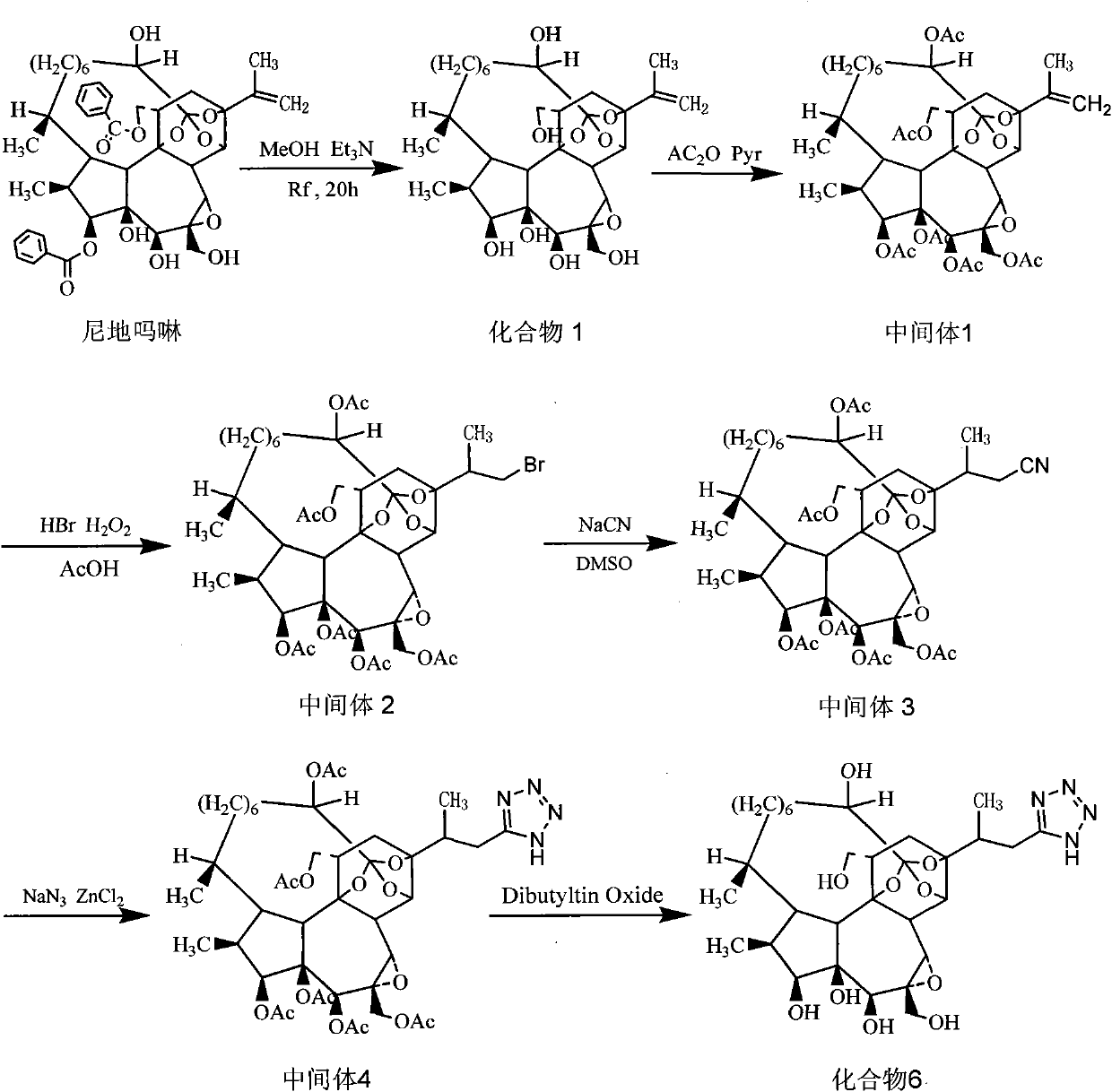
Antitumor drug (hydroxyl morpholine) and derivative thereof as well as preparation method and application thereof
An anti-tumor drug and pharmaceutical technology, applied in the field of medicine, can solve the problems of insignificant tumor cell inhibition, poor safety, and narrow therapeutic window, and achieve excellent anti-tumor activity and safety, wide therapeutic window, and broad anti-cancer spectrum Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0057] Embodiment 1 compound 1 and preparation method thereof, the structural formula of compound 1 is shown in (1)
[0058]
[0059] Take a dry 50ml eggplant-shaped bottle, add a magnetic stirring bar, 1.548g (2mmol) of nidimorph, add methanol: triethylamine: water (v: v: v) = 5: 1: 1 to dissolve a total of 21 ml of the solution , the reaction system was placed on a collector-type constant temperature heating magnetic stirrer to stir vigorously, the reaction system was sealed, and under the protection of dry nitrogen, the reaction was stopped after reflux at 70°C for 20 hours, and the reaction system was concentrated under reduced pressure at 50°C. Dried to obtain a brownish-yellow primary product, which was separated by silica gel column chromatography to obtain compound 1, a total of 0.46g, a yield of 40.6%, mp 150-152°C.
[0060] Elemental analysis found values: C, 63.54; H, 8.19.
[0061] Molecular formula (C 30 h 46 o 10 ) calculated: C, 63.58; H: 8.18.
[0062] ...
Embodiment 2
[0066] Embodiment 2 compound 6 (that is, oxydimorph) and preparation method thereof
[0067] (1) The preparation of intermediate 1, the structural formula of intermediate 1 is as follows:
[0068]
[0069] Take a dry 50ml eggplant-shaped bottle, add a magnetic stirring bar, 0.452g (0.8mmol) of compound 1, add 10ml of tetrahydrofuran solvent, 1ml of acetic anhydride, and 1ml of pyridine, and place the reaction system in a collector type constant temperature heating magnetic stirrer The reaction was stopped after 8 hours of closed and vigorous stirring at room temperature. Under reduced pressure at 45°C, spin out THF from the reaction bottle, add 20ml of anhydrous diethyl ether, stir vigorously at room temperature, a large amount of white solid precipitates, filter and dry to obtain intermediate 1, a total of 0.62g, yield 95.1%, mp 156~158°C .
[0070] Elemental analysis found values: C, 61.56; H, 7.16.
[0071] Molecular formula (C 42 h 58 o 16 ) calculated: C, 61.60; ...
Embodiment 3
[0109] Embodiment 3 compound 7 and preparation method thereof
[0110] use Figure 4 Represent the preparation technology of compound 7, the structural formula of compound 7 is as shown in (7),
[0111]
[0112] Take a dry 25ml eggplant-shaped bottle, add a magnetic stirring bar, 0.14g (0.16mmol) of intermediate 4, 0.12g of zinc acetate, add 15ml of anhydrous methanol, and seal the reaction system. Under the protection of dry nitrogen, the reaction system Stir vigorously on a collector-type constant-temperature heating magnetic stirrer, and stop the reaction after reflux at 70°C for 8 hours. The reaction solution is concentrated under reduced pressure at 50°C, and separated by column chromatography on silica gel to obtain a total of 0.03g of compound 7, with a yield of 25.8%, mp 176-179°C.
[0113] Elemental analysis found values: C, 60.06; H, 7.81; N, 8.95.
[0114] Molecular formula (C 31 h 48 N 4 o 9 ) calcd: C, 59.98; H, 7.79; N, 9.03.
[0115] Mass spectrum MS ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com