Medicinal-grade magnesium stearate and refining process thereof
A magnesium stearate, pharmaceutical grade technology, applied in the field of pharmaceutical grade magnesium stearate and its refining, can solve the problems of large container equipment, low production efficiency, high heavy metal content and sulfate content, and meet the requirements of low heavy metal The effect of content requirements, high product quality level, and simple preparation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0033] (2) Preparation of iron-modified sepiolite adsorbent
[0034] Take 3 parts of sepiolite fine powder 10g and place them in 100mL beakers respectively, add FeCl with mass fractions of 2.5%, 5.0%, and 10% in sequence 3 The solution was 50mL, the solid-liquid ratio was 1:5, the reaction was stirred for 30min, and the precipitation was allowed to stand for 24h, during which time it was stirred 7-8 times. Discard the supernatant, transfer the lower precipitate to a funnel, filter, and wash with water until the effluent does not contain Fe 3+ , Mg 2+ until. Take it out, put it in an oven at 150°C and bake it for more than 8 hours, smash it with a ceramic bowl, dry it, and crush it through a 100-mesh sieve to prepare 2.5%, 5.0% and 10% iron-modified sepiolite adsorbents. The iron-modified sepiolite adsorbent is placed in a sealed bag in a desiccator for standby.
[0035] Preparation of pharmaceutical grade high-purity magnesium stearate with low heavy metal content
[00...
Embodiment 1
[0049] The preparation method of embodiment 1 magnesium stearate
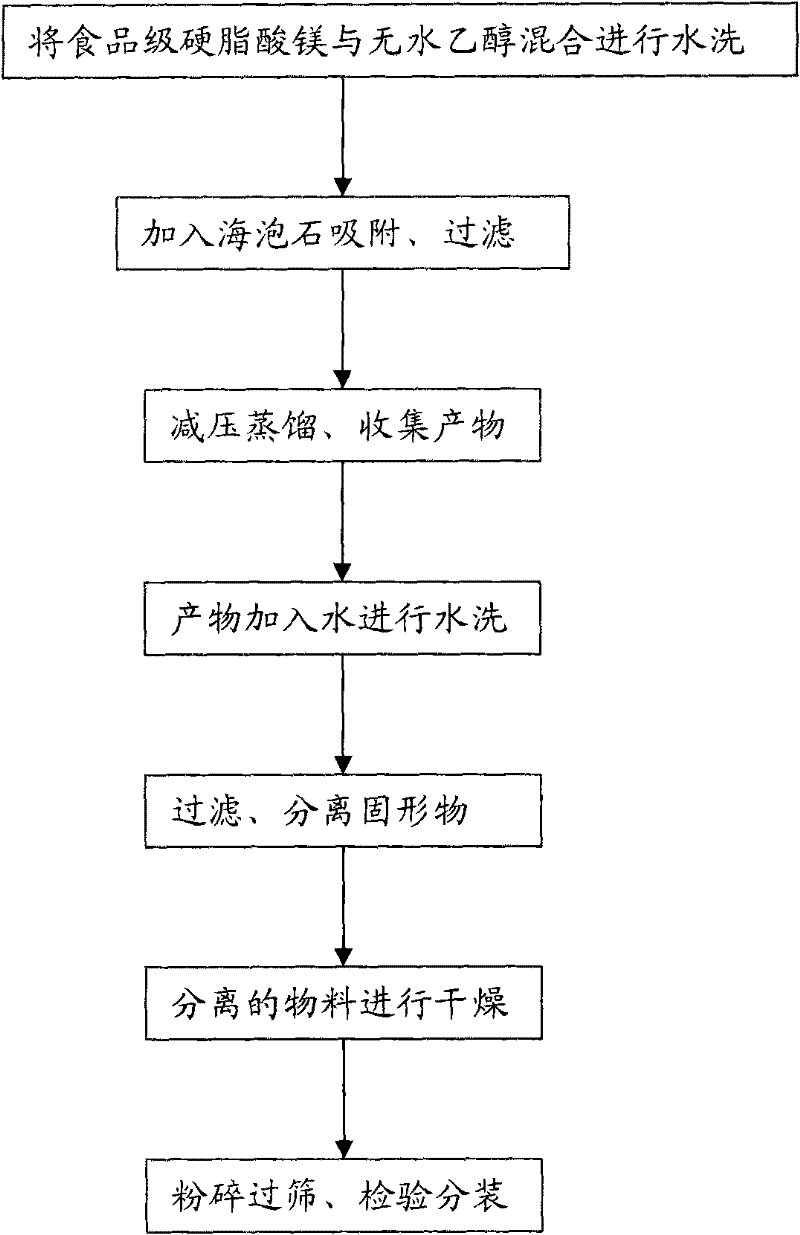
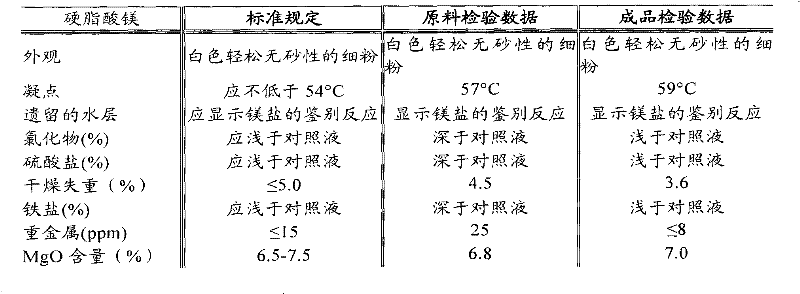
[0050] Add 100g of food-grade magnesium stearate into the dissolving tank, then add 1000ml of absolute ethanol into the dissolving tank, stir at a stirring speed of 120rpm for 20 minutes, and heat to 50°C to fully dissolve; add the obtained solution to 10g / L of iron-modified sepiolite adsorbent, oscillating for 2 hours, filtered; the filtrate was distilled under reduced pressure at 60°C to recover ethanol, and the product was collected; Stir at a stirring speed of 120rpm for 20 minutes to fully wash with water at a certain temperature, wherein the mixing ratio of magnesium stearate and purified water is 1:8 (g / ml); filter, separate the solids, and discard the filtrate; The raw materials were dried in an oven at 80° C. for 4 hours; the dried raw materials were crushed and sieved, inspected and packaged to obtain products. The magnesium stearate content of the pharmaceutical grade magnesium stearate prepared by...
Embodiment 2
[0054] Add 200g of food-grade magnesium stearate into the dissolving tank, then add 2000ml of absolute ethanol into the dissolving tank, stir at a stirring speed of 130rpm for 30 minutes, and heat to 60°C to fully dissolve; add the obtained solution to 20g / L of iron-modified sepiolite adsorbent, oscillating for 3 hours, filtered; the filtrate was distilled under reduced pressure at 70°C to recover ethanol, and the product was collected; Stir at a stirring speed of 150rpm for 30 minutes to fully wash with water at a certain temperature, wherein the mixing ratio of magnesium stearate and purified water is 1:8 (g / ml); filter, separate the solids, and discard the filtrate; The raw materials were dried in an oven at 85° C. for 5 hours; the dried raw materials were crushed and sieved, inspected and packaged to obtain products. The magnesium stearate content of the pharmaceutical grade magnesium stearate prepared by the method is 99.87%, and the heavy metal content is 5.1ppm, which ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com