Isoindolinone compound containing eneyne structure and synthetic method thereof
A technology of isoindolinone and compound, which is applied in the field of isoindolinone compound and its preparation, can solve the problems of high price and limited application of iodide, and achieve the effects of simple operation, high reaction yield and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
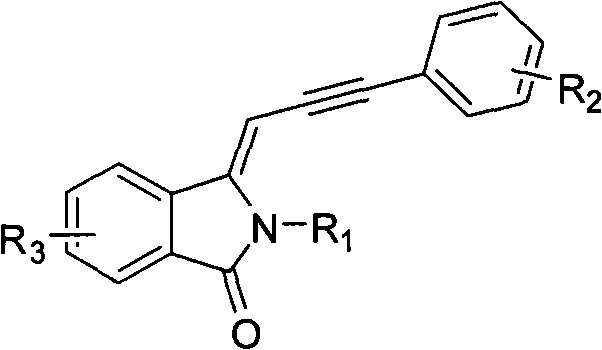
[0062] Example 1: Preparation of (Z)-3-(3-phenylprop-2-ynylmethine)isoindol-1-one
[0063] (Z)-3-(3-phenylprop-2-ynylmethine)isoindol-1-ketone adopts the following steps: 1. add 22.5 grams of (Z)-3- Bromomethylene isoindol-1-one, 10.2 g of phenylacetylene, 0.70 g of dichlorobis(triphenylphosphine)palladium, 0.19 g of cuprous iodide, and 90 ml of triethylamine were heated to 100°C under reflux. Use thin layer chromatography to track the reaction until the reaction raw material (Z)-3-bromomethyleneisoindol-1-one disappears; ②After the reaction is completed, filter with diatomaceous earth, and use a rotary evaporator to remove the solvent to obtain a crude product ; ③The crude product is purified by column chromatography (petroleum ether: ethyl acetate=6: 1) to obtain 22.3 grams of (Z)-3-(3-phenylprop-2-ynylmethine) isoindole- 1-Kone, 91% yield. Melting point: 204-206°C.
[0064] IR(KBr, cm -1 ): 3441, 3168, 1696, 1631, 1590, 1492, 1395, 918, 746. 1 H NMR (CDCl 3 , 500MHz):...
Embodiment 2
[0068] Example 2: Preparation of (Z)-3-(3-(4-fluorophenyl)prop-2-ynylmethine)isoindol-1-one
[0069] (Z)-3-(3-(4-fluorophenyl) prop-2-ynylmethine) isoindol-1-ketone adopts the following steps: 1. add 22.5 grams ( Z)-3-bromomethyleneisoindol-1-one, 14.4 grams of p-fluorophenylacetylene, 1.4 grams of dichlorobis(triphenylphosphine) palladium, 0.76 grams of cuprous iodide, 90 milliliters of triethylamine, Heat to reflux at 100°C. Track the reaction with thin-layer chromatography until the reaction raw material (Z)-3-bromomethyleneisoindol-1-one disappears; ②After the reaction, filter the system with diatomaceous earth, and remove the solvent with a rotary evaporator Obtain crude product; ③ crude product is purified with column chromatography (petroleum ether: ethyl acetate=6: 1), obtains 23.7 grams of (Z)-3-(3-(4-fluorophenyl) prop-2-ynyl Methylene) isoindol-1-one, the yield is 90%. Melting point: 231-233°C. IR(KBr, cm -1 ): 3448, 3183, 3056, 1702, 1638, 1595, 1506, 1470, 14...
Embodiment 3
[0074] Example 3: Preparation of (Z)-3-(3-(4-pentylphenyl)prop-2-ynylmethine)isoindol-1-one
[0075] (Z)-3-(3-(4-pentylphenyl) prop-2-ynylmethine) isoindol-1-one adopts the following steps: 1. add 22.5 grams of (Z)-3-Bromomethyleneisoindol-1-one, 24.1 g p-pentylphenylacetylene, 2.8 g dichlorobis(triphenylphosphine)palladium, 1.3 g cuprous iodide, 90 ml triethyl Amine, heated to reflux at 100°C. Track the reaction with thin-layer chromatography until the reaction raw material (Z)-3-bromomethyleneisoindol-1-one disappears; ②After the reaction, filter the system with diatomaceous earth, and remove the solvent with a rotary evaporator Obtain crude product; 3. crude product is purified by column chromatography (petroleum ether: ethyl acetate=6: 1), obtains 28.7 grams of (Z)-3-(3-(4-pentylphenyl) prop-2-yne methine) isoindol-1-one with a yield of 91%. Melting point: 171-173°C.
[0076] IR(KBr, cm -1 ): 3443, 3160, 3057, 2950, 2863, 2185, 1702, 1639, 1603, 1507, 1396, 1136, 94...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com