Use and preparation of indazole compound
A compound, indazole technology, applied in the field of medicine, can solve the problems of long synthesis process and low yield, and achieve the effects of high yield, cerebral blood vessel protection, and neurological dysfunction improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
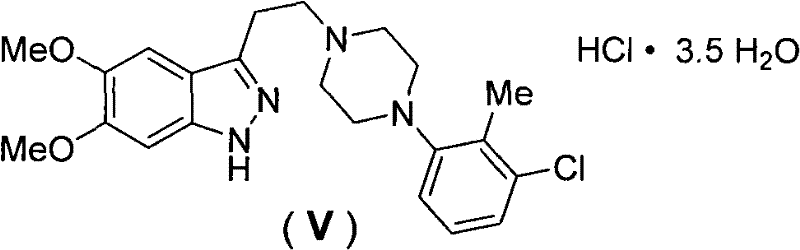
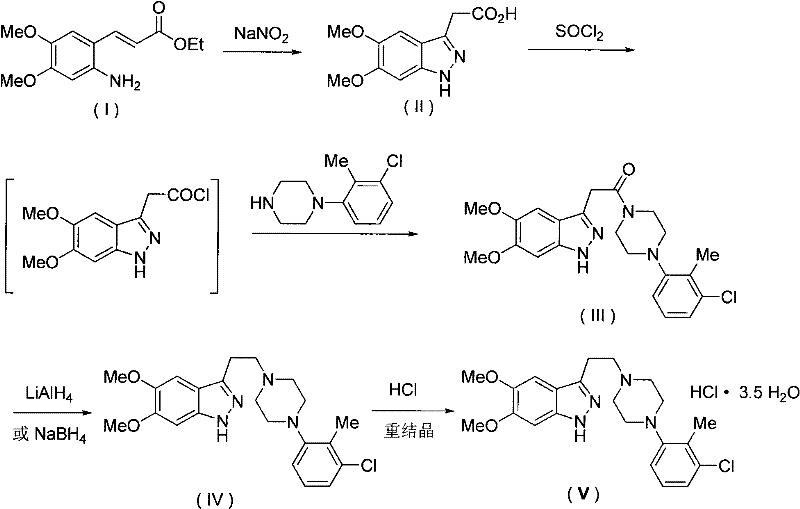
[0019] Example 1 Synthesis of 3-{2-[4N-(2-methyl-3-chlorophenyl)-1N-piperazinyl]ethyl}-5,6-dimethoxy-1H-ind by chemical synthesis azole hydrochloride
[0020] Step 1: Indazolylacetic acid synthesis
[0021] Put 669 mg of ethyl 2-amino-3,4-dimethoxycinnamate (I) into 3 ml of 2mol / L hydrochloric acid under ice-cooling conditions, and stir evenly. 1.5ml of 140g / L sodium nitrite aqueous solution was added dropwise thereto, and half an hour later, 4.5ml of 3.0mmol / L sodium sulfite aqueous solution was added and mixed thoroughly. Then add 6 mL of ethanol to it, stir and filter the crystals, wash with ethanol, dry under reduced pressure at 50°C for 4 hours, suspend it in 2mol / L 1.0mL hydrochloric acid, and stir at 60°C for half an hour. Adjust the pH to neutral with sodium acetate, collect the crystals by filtration, wash with water, and dry under reduced pressure at 50°C for 4 hours to obtain 248 mg of indazolylacetic acid (II).
[0022] Step 2: Synthesis of Indazolylacetamide
...
Embodiment 2
[0028] Example 2 Synthesis of 3-{2-[4N-(2-methyl-3-chlorophenyl)-1N-piperazinyl]ethyl}-5,6-dimethoxy-1H-ind by chemical synthesis Azole hydrochloride (V)
[0029] Step 1: Synthesis of Indazolylacetamide (III)
[0030] Get 4.724 g of the product indazole acid (II) obtained in step 1 in Example 1, add 50 mL of thionyl chloride therein, and after reflux for 1 hour, dissolve the residue in 100 mL of tetrahydrofuran, and add dropwise the solution containing 4.21g 1-1N-(2-methyl-3-chlorophenyl)piperazine in 10mL tetrahydrofuran solution, stirred at room temperature for 3 hours, then quenched by adding saturated aqueous sodium bicarbonate solution, extracted 4 times with 100mL ethyl acetate each time , dried over anhydrous sodium sulfate, filtered, and the filtrate was concentrated under reduced pressure to obtain 8.2 g of the crude product of indazolylacetamide (III), with a yield of 96%.
[0031] Step 2: 3-{2-[4N-(2-Methyl-3-chlorophenyl)-1N-piperazinyl]ethyl}-5,6-dimethoxy-1H-in...
Embodiment 3
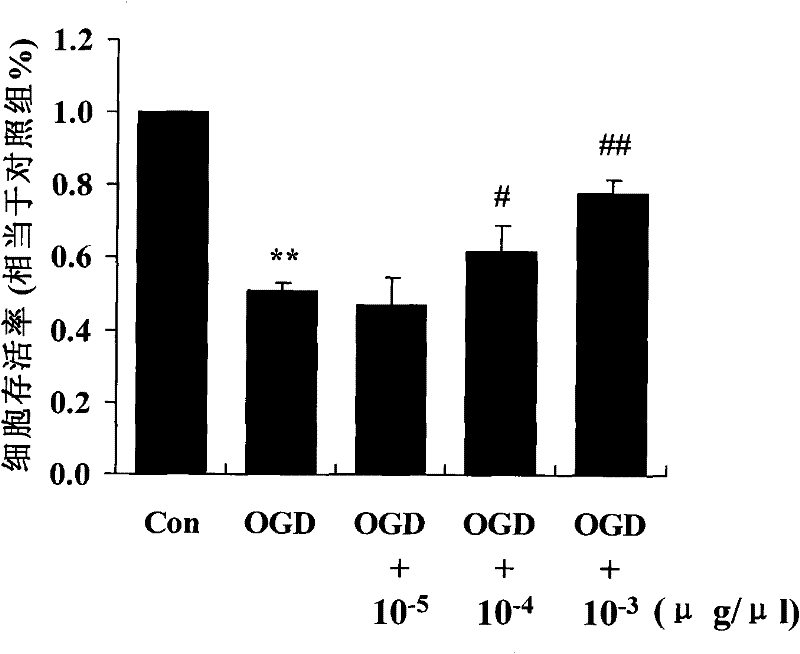
[0037] Example 3 3-{2-[4N-(2-methyl-3-chlorophenyl)-1N-piperazinyl]ethyl}-5,6-dimethoxy-1H-indazole hydrochloride Protective effect on vascular endothelial cell injury under hypoxia and low glucose oxidative stress injury
[0038] Drugs and Reagents
[0039] High-glucose DMEM and high-glucose DMEM medium were purchased from GIBICO Company in the United States, fetal bovine serum and newborn calf serum were purchased from Hangzhou Sijiqing Bioengineering Materials Research Institute, type I collagenase and tetrazolium blue were products of Sigma Company, and other reagents Both domestic and imported analytical reagents were used. The human umbilical vein endothelial EA.hy926 cell line used for cell culture in the experiment is an immortalized cell line.
[0040] cell culture
[0041] Human umbilical vein endothelial EA.hy 926 cells were cultured in high-glucose DMEM (containing 10% inactivated domestic fetal bovine serum) at 37°C, saturated air humidity, containing 5% CO 2 ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| weight | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com