Organic electroluminescent iridium coordination compound and preparation method and application thereof
An iridium complex, electroluminescence technology, applied in the directions of organic chemistry, luminescent materials, chemical instruments and methods, etc., can solve the problems of difficult synthesis of electroluminescent iridium complexes, low electroluminescence efficiency, poor solution processing performance, etc., Achieve the effect of improving carrier injection and transport capabilities, good host material properties, and promoting energy transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach 1
[0026] Specific Embodiment 1: The organic electroluminescent iridium complex of this embodiment uses trivalent iridium as the ion as the central ion, and alkyl-substituted phenyl benzimidazole modified by a hole-transporting group as the ligand, and its general formula is as follows:
[0027]
[0028] Wherein, Ar is a hole-transporting group of carbazole, a carbazole derivative or a triarylamine derivative, m=0~3, n=2~6, m and n are both positive integers, and the structural formula of the ligand is:
[0029]
[0030] In the organic electroluminescent iridium complex of this embodiment, when m is not 0, the alkyl group substituted by the hole transport group is at the 2-position, 3-position, 4-position and 5-position on the phenyl group of phenylbenzimidazole Substitutions are made at one, two or three of the four substituted positions of a position.
[0031] In this embodiment, Ar is a hole transport group, and when Ar is a carbazole derivative, Ar is 3,6-alkylcarbazole...
specific Embodiment approach 2
[0033] Specific embodiment two: the difference between this embodiment and specific embodiment one is that Ar is carbazole, m=0, and the ligand is 1-(carbazole-N-alkyl)-2-phenylbenzimidazole, whose structural formula as follows:
[0034] Wherein n=2~6. Other parameters are the same as in the first embodiment.
[0035] The organic electroluminescent iridium complex of this embodiment is a 1-(carbazole-N-alkyl)-2-phenylbenzimidazole iridium complex (referred to as Ir(CzPhBI) 3 ).
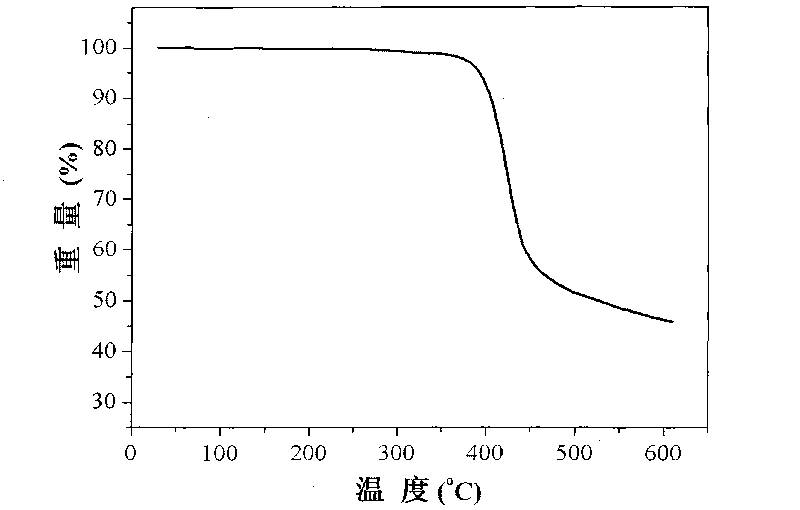
[0036] This embodiment is for Ir(CzPhBI) 3 Carry out thermogravimetric analysis, the thermogravimetric curve that test obtains is as follows figure 1 shown in . Depend on figure 1 It can be seen that the Ir(CzPhBI) of this embodiment 3 Its thermal decomposition temperature is 402°C, and it has good thermal stability.
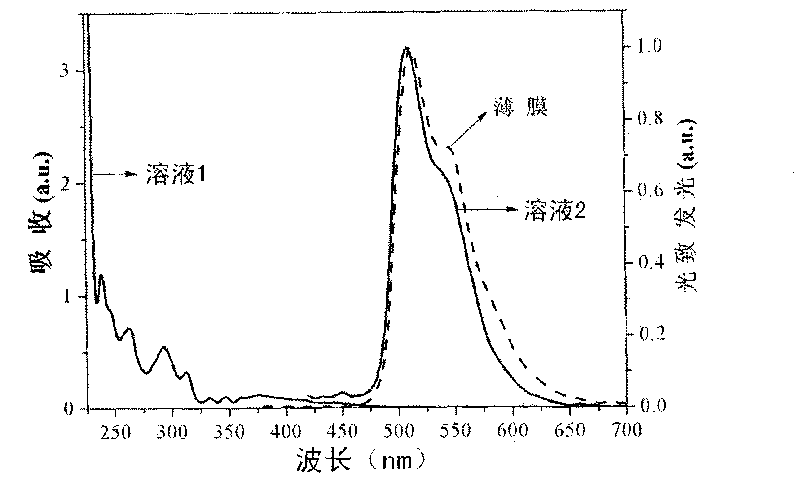
[0037] In this embodiment, Ir(CzPhBI) 3 It was dissolved in dichloromethane solvent to form a solution, and the solution was tested by ultraviolet fluorescence spectrum, and ...
specific Embodiment approach 3
[0038] Specific embodiment 3: The difference between this embodiment and specific embodiment 1 is that Ar is carbazole, m=1, and the alkyl group modified by the hole transport group is substituted on the 2-position of the phenyl group of phenylbenzimidazole, The ligand is 1-(carbazole-N-alkyl)-2-(2-carbazole-N-alkoxyphenyl)benzimidazole, and its structural formula is as follows:
[0039] Wherein n=2~6. Other parameters are the same as in the first embodiment.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com