Method for preparing benzoyl-flavone
A technology for benzoyl flavonoids and dihydroxyacetophenone, which is applied in the field of flavonoids, can solve the problems of low yield, complicated extraction methods and the like, and achieves the effects of high yield, easy operation and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
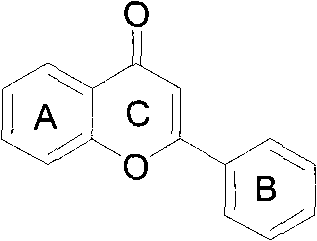
[0016] The synthesis of embodiment 1,7-benzoylflavone:
[0017] In a 250mL single-necked bottle, dissolve 30g of resorcinol in 30mL of glacial acetic acid, add 50g of anhydrous zinc chloride, and react for 5min under 150W microwave radiation to obtain a brown viscous substance, which is poured into 100mL of water , precipitated an orange precipitate, separated by suction filtration, washed 3 times with water, and dried at 80°C for 2h to 3h to obtain 37.16g of product A (2,4-dihydroxyacetophenone), with a yield of 87.3%, m.p.145 ~147°C. Take 15g of product A and add 100g of anhydrous potassium carbonate, dissolve it in 200mL of acetone, put it in a 500mL three-neck flask, heat it in a water bath (temperature 60°C to 80°C) under magnetic stirring, add dropwise 25mL of benzoyl chloride, and react After 15 hours, a bright yellow precipitate was obtained. Cool the reaction mixture to room temperature, filter, wash with a small amount of acetone three times, the filter cake is bri...
Embodiment 2
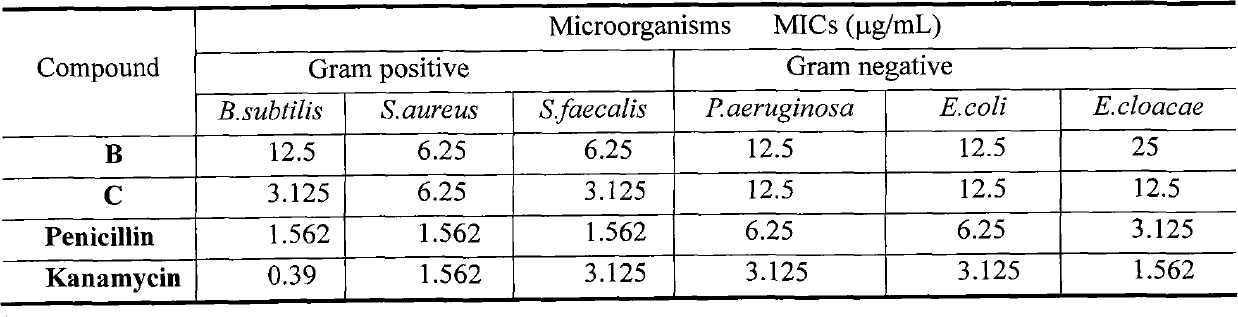
[0018] Example 2, 7-benzoyl flavones to Bacillus subtilis (B.subtilis), Staphylococcus aureus (S.aureus), Enterococcus faecalis (S.faecalis), Pseudomonas aeruginosa (P.aeruginosa), large intestine The role of Bacillus (E.coli) and Enterobacter cloacae (E.cloacae):
[0019] Method: MTT method, cultured Bacillus subtilis (B.subtilis), Staphylococcus aureus (S.aureus), Enterococcus faecalis (S.faecalis), Pseudomonas aeruginosa (P.aeruginosa), Escherichia coli (Escherichia coli) .coli) and Enterobacter cloacae (E.cloacae) strains were diluted to 2×10 4 per mL, dispensed in 96-well plates (0.2 mL / well). Set penicillin and kanamycin as reference control group, DMSO control group and 16 tested compounds with different concentrations, 10 μL per well. Three parallel wells were set up in each group, cultured in a constant temperature incubator at 37°C for 24 hours, injected with 5 μL / well of MTT solution (2 mg / mL), and cultured for another 4 hours. Take out the culture plate, add SDS...
Embodiment 3
[0024] Embodiment 3, the effect of 7-benzoylflavone on vasodilation:
[0025] Methods: Rats were sacrificed by bleeding from the common carotid artery, and the thoracic aorta was quickly removed and placed in a 95% O 2 +5%CO 2 In the ice-cold Krebs solution mixed with gas, the blood vessels were cut into vascular rings about 3-4mm long after carefully removing the surrounding connective tissue. Put the vascular ring into the bath containing Krebs solution, and continuously pass it with 95% O 2 +5%CO 2 The mixed gas, adjust the resting tension to 2g. Stable at 37°C for 90 minutes, during which time the liquid was changed every 15 minutes, using 60 mmol·L -1 The KCl preconstricts blood vessels, and observes the effect of the drug after reaching the plateau value.
[0026] Experiments show that substance C has obvious relaxation effect on blood vessels of rats, and the experimental results are shown in Table 2.
[0027] Table 2 Substances B and C have vasodilation effects o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com