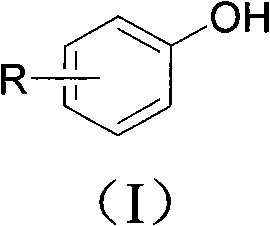
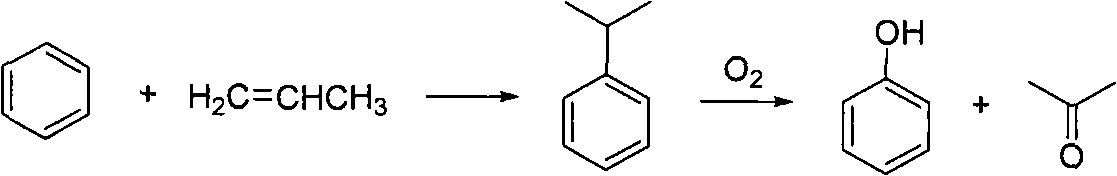
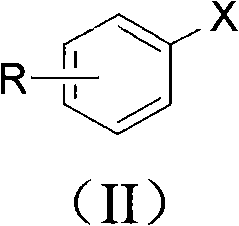Phenolic compound synthesizing method taking water as solvent
A technology of phenolic compounds and synthesis methods, which is applied in the field of synthesis and preparation of chemical products, can solve problems such as pollution and high yields, and achieve the effects of cheap and easy-to-obtain reaction raw materials, high yields, and low environmental pollution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Add 7 mg of cuprous oxide, 120 mg of sodium hydroxide, 12 mg of 2-pyridine formaldehyde oxime, 65 mg of tetra-n-butylammonium bromide and 201 mg of o-bromobenzoic acid into a Schrank test tube equipped with a magnetic stirring bar. After the Schrank test tube was evacuated twice, it was filled with nitrogen, and 1 mL of water was added to the Schrank test tube at room temperature, then the Schrank test tube was sealed and placed in an oil bath at 100°C under nitrogen atmosphere. Under pressure, react for 48 hours. After the reaction was completed, cool to room temperature, then add 2 mL of 1 mol / L hydrochloric acid for acidification (pH 2-3), extract 3 times with ethyl acetate, 2 mL each time, the combined organic phase was concentrated, and the residue Purification was carried out through a silica gel column to obtain 124 mg of o-hydroxybenzoic acid with a yield of 90%.
[0057] Product o-hydroxybenzoic acid: 1 H NMR (DMSO-d 6 , 300MHz, ppm) δ11.8(s, br, 1H), 7.79(d...
Embodiment 2
[0059] 19 mg of cuprous iodide, 168 mg of potassium hydroxide, 12 mg of 2-pyridine formaldehyde oxime, 65 mg of tetra-n-butylammonium bromide and 239 mg of 1-chloro-4-iodobenzene were added to a Schrank test tube equipped with a magnetic stirring bar. After the Schrank test tube was evacuated twice, it was filled with nitrogen, and 1 mL of water was added to the Schrank test tube at room temperature. Under pressure, react for 48 hours. After the reaction was completed, cool to room temperature, then add 2 mL of 1 mol / L hydrochloric acid for acidification (pH 2-3), extract 3 times with ethyl acetate, 2 mL each time, the combined organic phase was concentrated, and the residue Purification was carried out through a silica gel column to obtain 78 mg of 4-chlorophenol with a yield of 61%.
[0060] Product 4-chlorophenol: 1 H NMR (CDCl 3 , 300MHz, ppm) δ7.19(d, 2H, J=7.9Hz), 6.77(d, 2H, J=7.9Hz), 5.13(s, br, 1H). 13 C NMR (CDCl 3 , 75MHz, ppm) δ154.1, 129.6, 125.8, 116.8.ESI-M...
Embodiment 3
[0062] 19 mg of cuprous iodide, 450 mg of cesium hydroxide, 12 mg of 2-pyridine formaldehyde oxime, 65 mg of tetra-n-butylammonium bromide and 239 mg of 1-chloro-4-iodobenzene were added to a Schrank test tube equipped with a magnetic stirring bar. After the Schrank test tube was evacuated twice, it was filled with nitrogen, and 1 mL of water was added to the Schrank test tube at room temperature. Under pressure, react for 48 hours. After the reaction was completed, cool to room temperature, then add 2 mL of 1 mol / L hydrochloric acid to acidify (pH 2-3), extract 3 times with ethyl acetate, 2 mL each time, the combined organic phase was concentrated, and the residue Purification was carried out through a silica gel column to obtain 85 mg of 4-chlorophenol with a yield of 66%.
[0063] Product 4-chlorophenol: 1 H NMR (CDCl 3 , 300MHz, ppm) δ7.19(d, 2H, J=7.9Hz), 6.77(d, 2H, J=7.9Hz), 5.13(s, br, 1H). 13 C NMR (CDCl 3 , 75MHz, ppm) δ154.1, 129.6, 125.8, 116.8.ESI-MS[M-H] - ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com