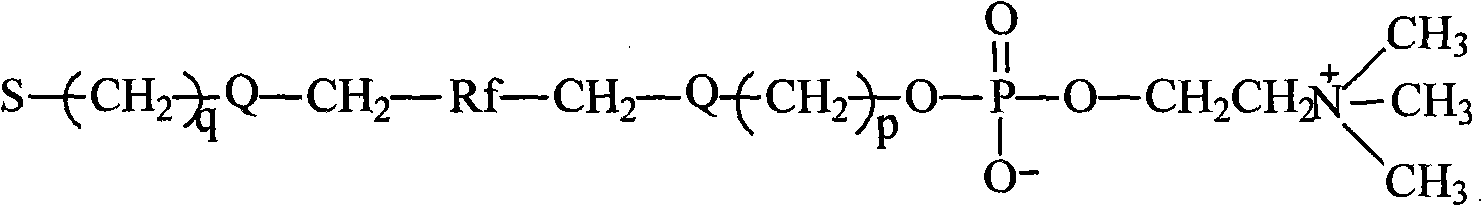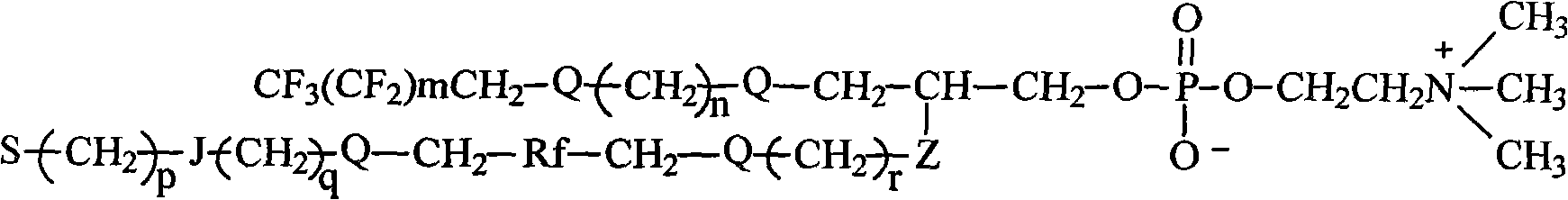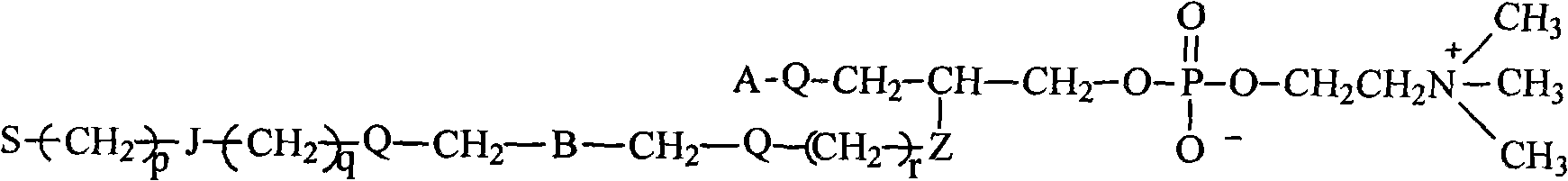Fluorocarbon chain or/and hydrocarbon chain containing phosphatidylcholine blocking agent with hydroxyl or amino at tail end and polyurethane material for blocking same
A technology for hydrocarbon chain phosphatidyl choline and polyurethane materials, which is applied in the field of polyurethane materials and can solve the problems of inability to carry out secondary processing, decreased mechanical properties of materials, poor compatibility and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0071] What this embodiment prepared was HDFOPC, and the codes used in this embodiment are respectively:
[0072] HDFDAC: (10-(1-carboxymethoxy)-2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9-hexadecafluorodecane Oxy)acetic acid
[0073] HDFOOL: 2-(2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9-hexadecafluoro-10-(2-hydroxyethoxy )decyloxy)ethanol
[0074] ACDFOOL: 2-(2,2,3,3,4,4,5,5,6,6,7,7,8,8,9,9-hexadecafluoro-10-(2-hydroxyethoxy )decyloxy)ethyl acetate
[0075] COP: Ethylene cyclophosphoryl chloride.
[0076] The method and steps for preparing HDFOPC are as follows:
[0077] Step 1: Synthesis of HDFOOL
[0078] Dissolve 2 grams of HDFDAC (3.46mmol) [see patent CN1569917A for the preparation method] in 30ml of anhydrous tetrahydrofuran, lower the temperature to about -15°C, add 0.95ml of N-methylmorpholine (8.65mmol), and then add 0.95ml of chlorine Isobutyl formate (7.3mmol), after reacting for 15 minutes, add excess sodium borohydride, after reacting for 2 hours, add a small amount of water to ...
Embodiment 2
[0090] What this embodiment prepared was ADDPC, and the codes used in this embodiment were respectively:
[0091] (BOC) 2 O: tert-butoxycarbonic anhydride
[0092] BOC-Serine: 2-(tert-butoxycarbonylamino)-3-(hydroxy)propionic acid
[0093] BDPA: 2-(tert-butoxycarbonylamino)-3-(decyloxy)propionic acid
[0094] BDOHP: 2-(tert-butoxycarbonylamino)-3-(decyloxy)propanol
[0095] ACDOHPC: 2-Amino-3(decyloxy)propylphosphatidylcholine
[0096] HOSu: N-Hydroxysuccinimide
[0097] BSA: 10-(2-(tert-butoxycarbonylamino)ethylamino)-10-oxodecanoic acid
[0098] BSSI: Succinimidyl 10-(2-(tert-butoxycarbonylamino)ethylamino)-10-oxodecanoate
[0099] BDDPC: 2-(10-(2-(tert-butoxycarbonylamino)ethylamino)-10-oxodecanylamido)-3-decyloxypropylphosphatidylcholine
[0100] ADDPC: 2-(10-(2-aminoethylamino)-10-oxydecylamino)-3-decyloxypropylphosphatidylcholine The method and steps for preparing ADDPC are as follows:
[0101] Step -: Synthesis of BOC-Serine
[0102] Dissolve 30 grams (0.2857mo...
Embodiment 3
[0133] What this embodiment prepared was ADFPC, and the codes used in this embodiment were respectively:
[0134]BAEFAC: 2-(10-(2-(2-(tert-butoxycarbonylamino)ethylamino)-2-oxyethoxy)-2,2,3,3,4,4,5,5, 6,6,7,7,8,8,9,9-hexadecafluorodecyloxy)acetic acid
[0135] ACDFOPC: ACDOHPC: 2-Amino-3(decyloxy)propylphosphatidylcholine
[0136] ADFPC: 2-(2-(10-(2-(2-aminoethylamino)-2-oxyethoxy)-2,2,3,3,4,4,5,5,6,6, 7,7,8,8,9,9-Hexadecafluorodecyloxy)acetamido)-3-(decyloxy)propylphosphatidylcholine
[0137] DCC: Cyclohexylcarbodiimide DCU: N,N-Dicyclohexylurea
[0138] The method and steps for preparing ADFPC are as follows:
[0139] Step 1: Synthesis of BAEFAC
[0140] Dissolve 5 grams (8.65 mmol) of HDFDAC [see patent CN1569917A for the preparation method] in 100 ml of anhydrous tetrahydrofuran, lower the temperature to about -10°C, add 1.14 ml (10.4 mmol) of N-methylmorpholine and 1.13 ml ( 8.65mmol) of isobutyl chloroformate, after 10 minutes of reaction, 1.38 grams (8.65mmol) of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com