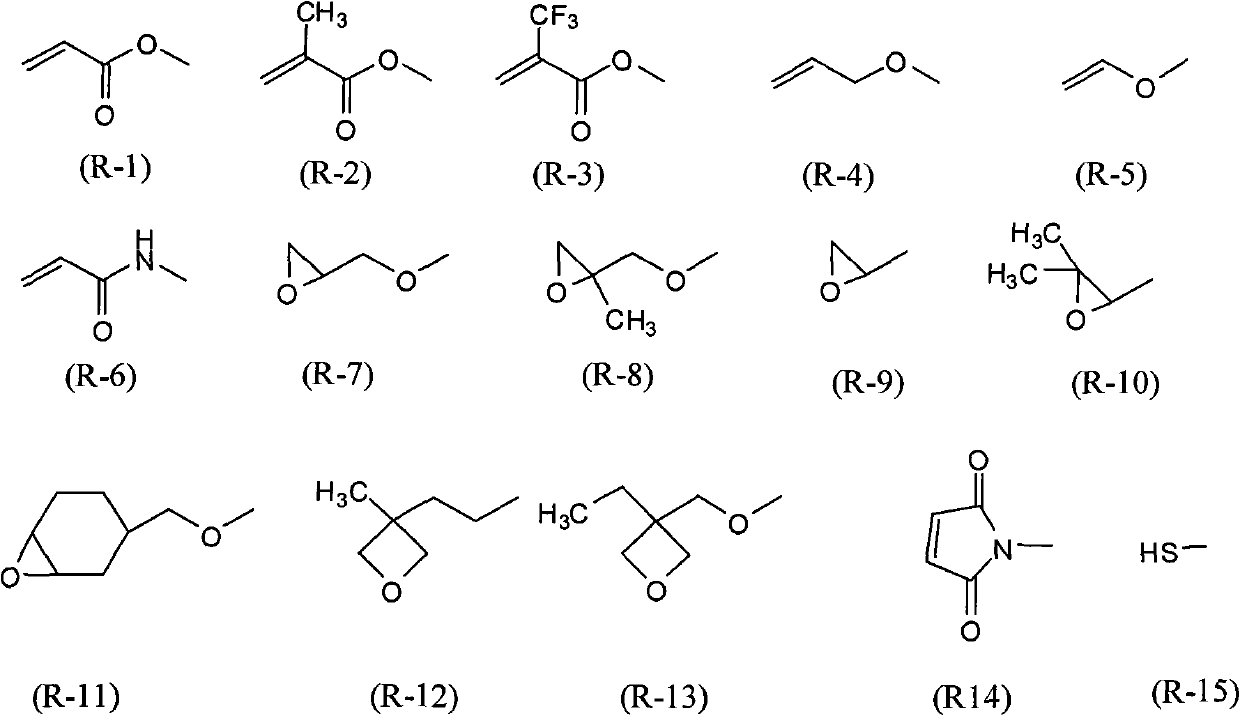
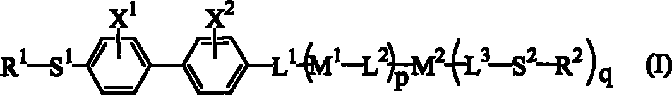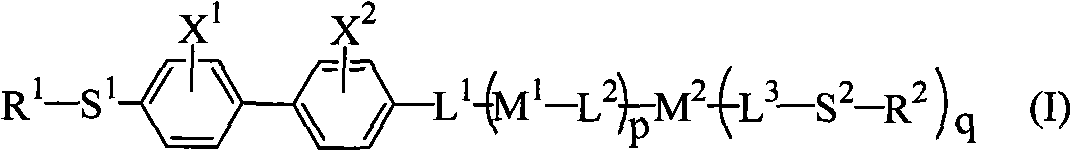Polymerizable biphenyl compound
A technology of polymeric compounds and phenylene, applied in the fields of organic chemistry, nonlinear optics, chemical instruments and methods, etc., can solve the problems of low solubility, low heat resistance and mechanical strength of polymeric compounds, etc., Achieve excellent solubility, wide temperature range of liquid crystal phase, and high heat resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0118] 10 g (40.1 mmol) of 4-bromo-4′-hydroxybiphenyl, 6.2 g (48.2 mmol) of tert-butyl acrylate, and 4.8 g of triethylamine were placed in a reaction vessel equipped with a stirring device, a cooler, and a thermometer. (48 mmol), 530 mg of palladium acetate, and 300 ml of dimethylformamide, and the reaction was carried out by heating the reactor to 100° C. under a nitrogen atmosphere. After completion of the reaction, ethyl acetate and THF were added, and the organic layer was washed with 10% aqueous hydrochloric acid, pure water, and saturated brine. After distilling off the solvent, purification was carried out with a silica gel column in a double amount (weight ratio) to obtain 11 g of the compound represented by the formula (1).
[0119] [chem 25]
[0120]
[0121] Then, 3 g (10.1 mmoles) of the compound represented by the above-mentioned formula (1), 1 g (11 mmoles) of acryloyl chloride, and 50 ml of dichloromethane were charged into a reaction vessel equipped with a ...
Embodiment 2
[0134] In the autoclave container equipped with stirring device, the intermediate 4.5g (15.2 mmoles) of embodiment 1 synthesis shown in the charging formula (1), 5% palladium carbon 250mg, tetrahydrofuran 50ml, ethanol 5ml, at 0.3MPa The reduction reaction was carried out in a hydrogen atmosphere (room temperature, 8 hours). After filtering the reaction liquid, the reaction solvent was distilled off to obtain 4.5 g of the compound represented by the formula (5).
[0135] [chem 29]
[0136]
[0137] Then, 4.5 g (15.1 mmoles) of the compound represented by the above-mentioned formula (5), 1.6 g (18 mmoles) of acryloyl chloride, and 50 ml of dichloromethane were charged into a reaction vessel equipped with a stirring device, a cooler, and a thermometer. The reactor was cooled to below 5°C under nitrogen atmosphere. Then, 1.8 g (18 mmol) of triethylamine was slowly added dropwise. After completion of the dropwise addition, the reaction was carried out at below 20° C. for 3 h...
Embodiment 3
[0151] 12.8 g (96 mmol) of aluminum trichloride and 100 ml of dichloromethane were added to a reaction vessel equipped with a stirring device, a cooler, and a thermometer, followed by stirring. Then, 8.4 g (110 mmol) of acetyl chloride was slowly added dropwise over 90 minutes, and further, 80 ml of a dichloromethane solution of 20 g (80 mmol) of 4-bromo-2-fluorobiphenyl was slowly added dropwise over 2 hours. After the dropwise addition was completed, stirring was continued for 2 hours to complete the reaction. The reaction solution was slowly poured into 500 ml of ice water, extracted with dichloromethane, and the organic layer was washed with pure water and saturated brine. After distilling off the solvent, drying was performed to obtain 23 g of a compound into which an acetyl group was introduced. Next, 23 g of an acetyl group-introduced compound and 300 ml of formic acid were placed in a reaction vessel equipped with a stirring device, a cooler, and a thermometer, and 20...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com