Method and device for preparing high-purity ruthenium
A high-purity, absorption device technology, used in ion implantation plating, coating, metal material coating processes, etc., can solve the problems of long oxidative distillation time, high operating costs, difficult removal of iron and silicon, and achieve oxidative distillation. The effect of short time, improved purity and high ruthenium extraction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0075] (1) One-stage oxidation and vacuum distillation to catch osmium.
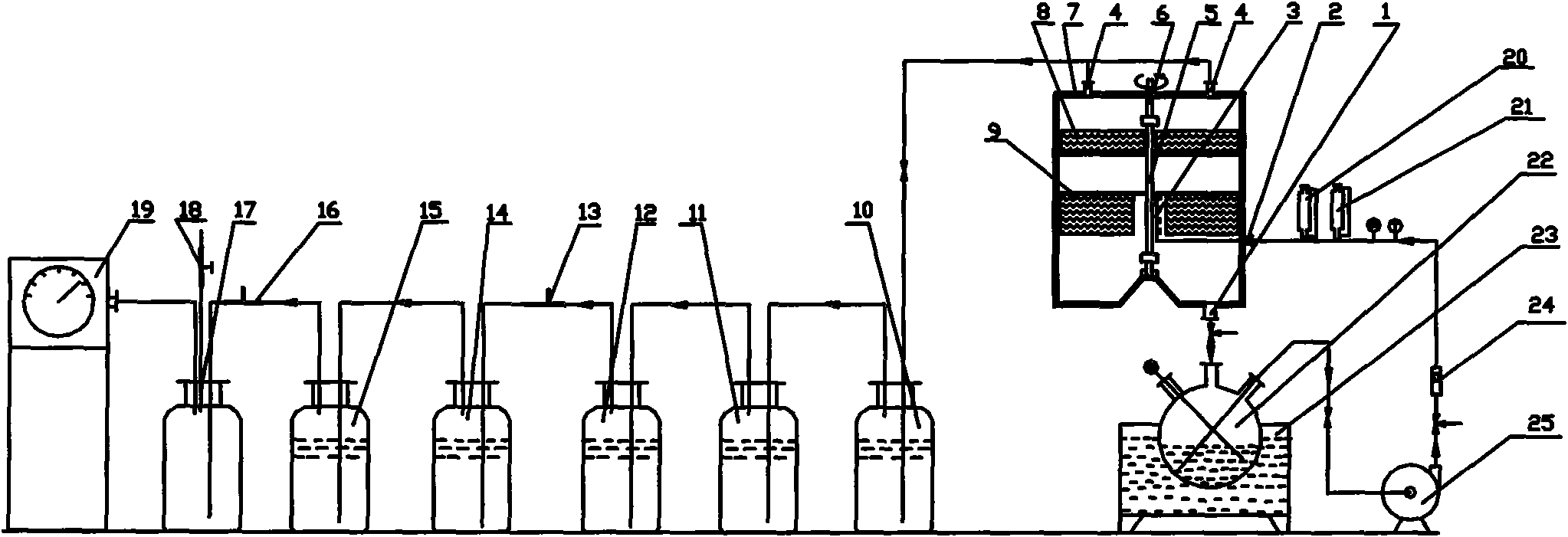
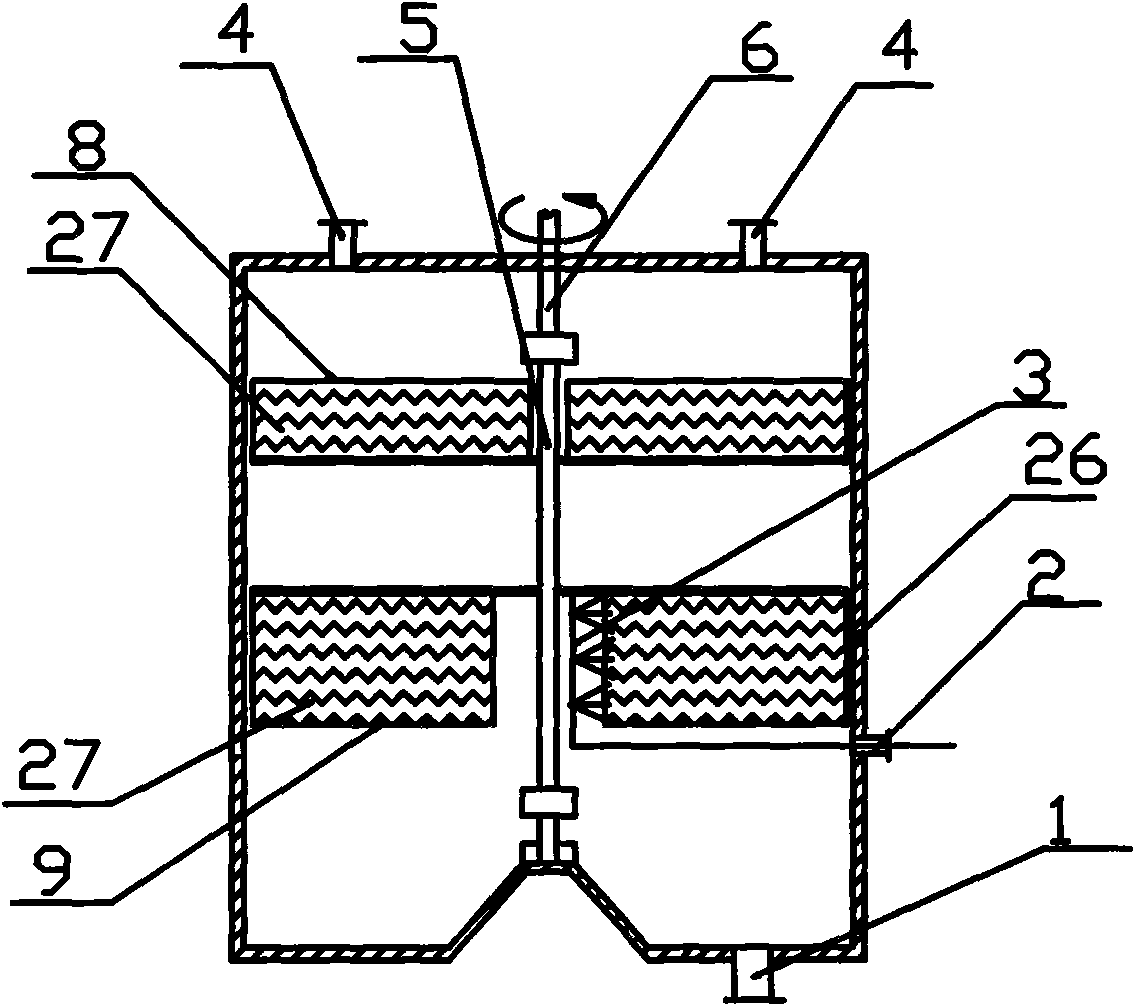
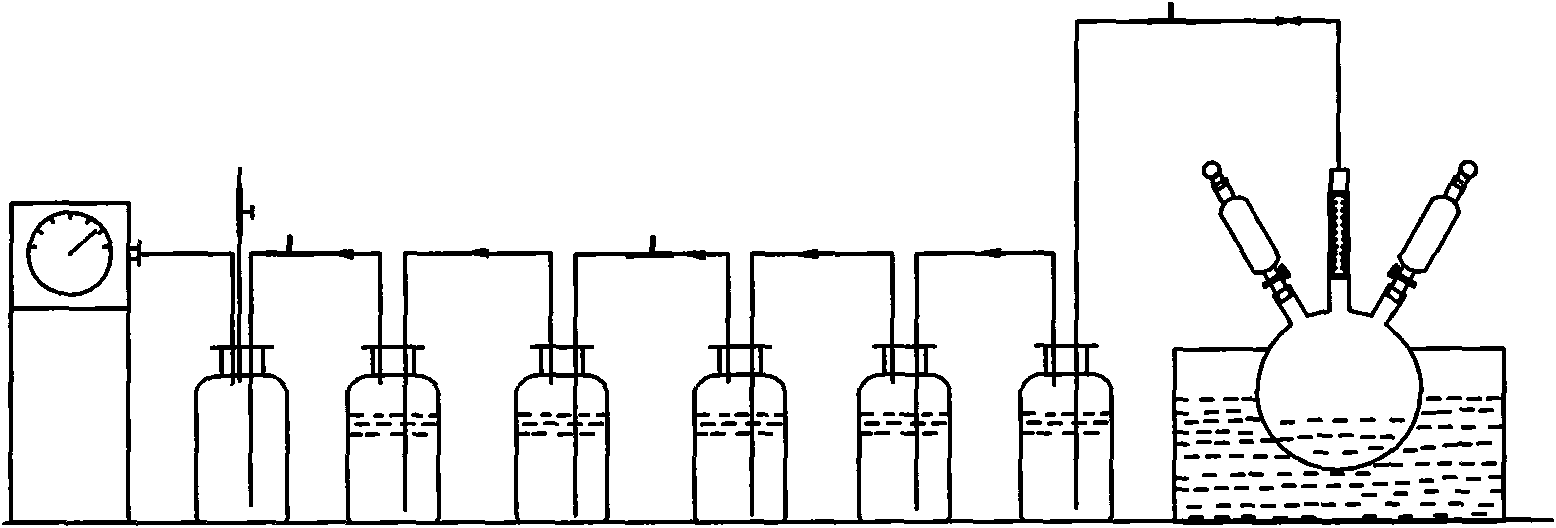
[0076] Put 1.5L ruthenium hydrochloric acid absorbing stock solution containing ruthenium 31.53g / L in the quartz flask (22), heat the ruthenium solution to 80°C by the oil bath heater (23), turn on the vacuum pump (19), and pass through the vacuum two-way valve (18) Adjust the internal vacuum to about 20KPa, turn on the rotary packing bed (7), and adjust the rotor speed of the rotary packing bed to 2000r / min through the frequency converter. (25) the ruthenium solution in the quartz flask (22) is regulated as 0.5m through liquid flowmeter (24) 3 / h enters the central liquid distributor (3) of the rotating packed bed from the liquid inlet pipe (2), and simultaneously from the constant pressure funnel (20) on the liquid inlet pipeline (2) according to the reaction equivalent multiple (due to the 3M In the hydrochloric acid system, ruthenium mainly exists as tetravalent, and a small amount of ruthenium exis...
Embodiment 2
[0090] (1) One-stage oxidation and vacuum distillation to catch osmium.
[0091] The one-stage oxidative vacuum distillation for removing osmium is basically the same as in Example 1, except that the rotational speed of the rotating packed bed rotor is adjusted to 1200 r / min.
[0092] (2) Two-stage oxidation and vacuum distillation to extract ruthenium.
[0093] The extraction of ruthenium by two-stage oxidation and vacuum distillation is basically the same as in Example 1, except that the rotor speed of the rotating packed bed is adjusted to 2000 r / min, and the reaction temperature is adjusted to 80°C.
[0094] (3) Repeat oxidation distillation to extract ruthenium.
[0095] Repeat once oxidative distillation to extract ruthenium. The operating conditions are completely the same as the two-stage oxidative vacuum distillation in Example 2 to extract ruthenium. The results of analysis of impurity elements in the three ruthenium hydrochloric acid absorption solutions are shown...
Embodiment 3
[0103] (1) One-stage oxidation and vacuum distillation to catch osmium.
[0104] One-stage oxidative vacuum distillation to remove osmium The operating conditions are completely the same as in Example 1.
[0105] (2) Two-stage oxidation and vacuum distillation to extract ruthenium.
[0106] The operating conditions for extracting ruthenium by two-stage oxidation and vacuum distillation are completely the same as in Example 1.
[0107] (3) Repeat oxidation distillation to extract ruthenium.
[0108] Repeat once oxidative distillation to extract ruthenium operating condition completely with embodiment 1. The results of analysis of impurity elements in the three ruthenium hydrochloric acid absorption solutions are shown in Table 2.
[0109] (4) Ammonium chloride crystallization precipitates ruthenium.
[0110] The operating conditions for ammonium chloride crystallization and precipitation of ruthenium are basically the same as in Example 1, except that the holding temperatur...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com