Anhydrous high-purity rare earth fluoride and preparation method thereof
A rare earth fluoride, high water technology, applied in rare earth metal compounds, chemical instruments and methods, inorganic chemistry, etc., can solve the problems of difficult filtration and washing, long precipitation and clarification time, and high content of non-rare earth impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
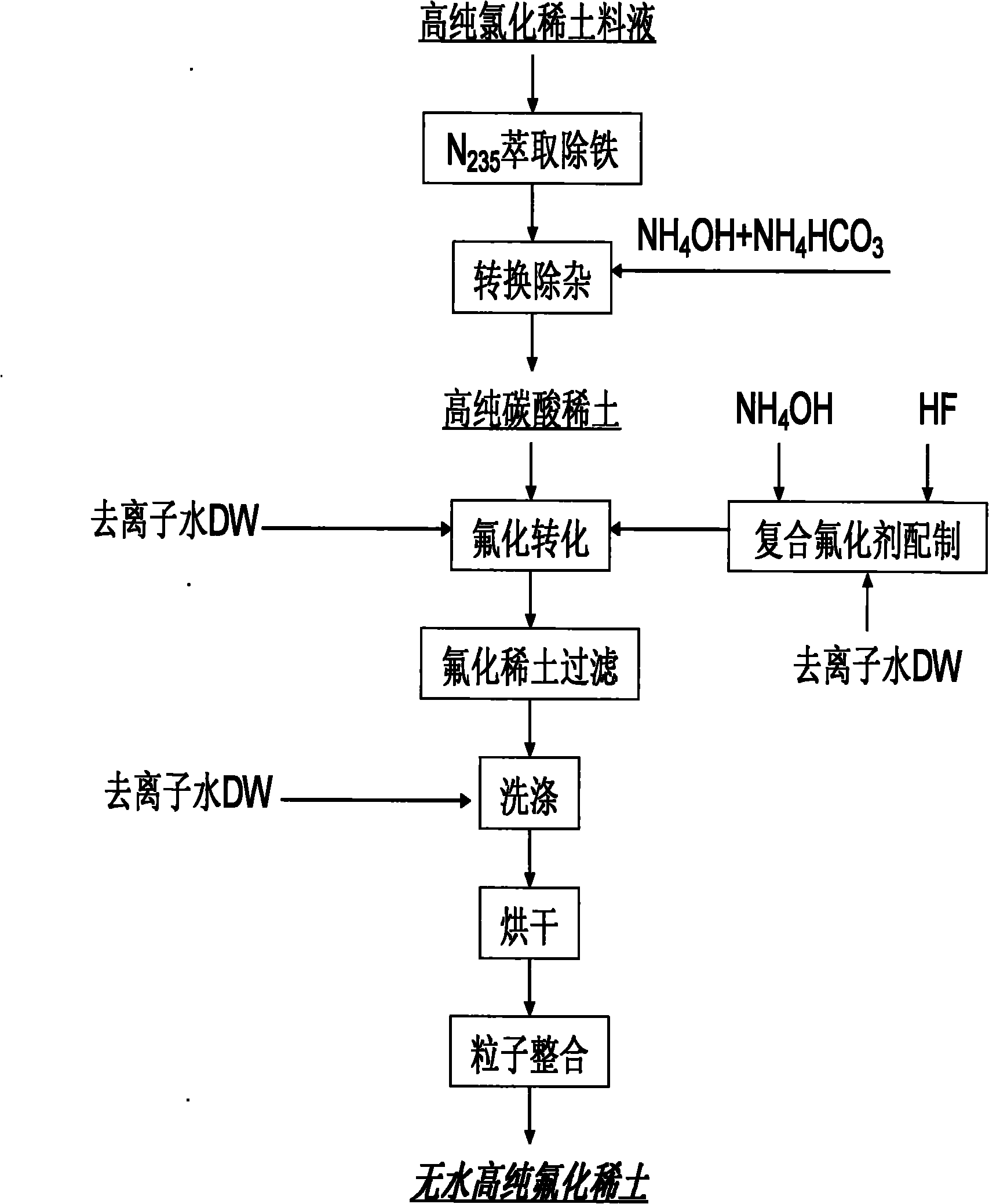
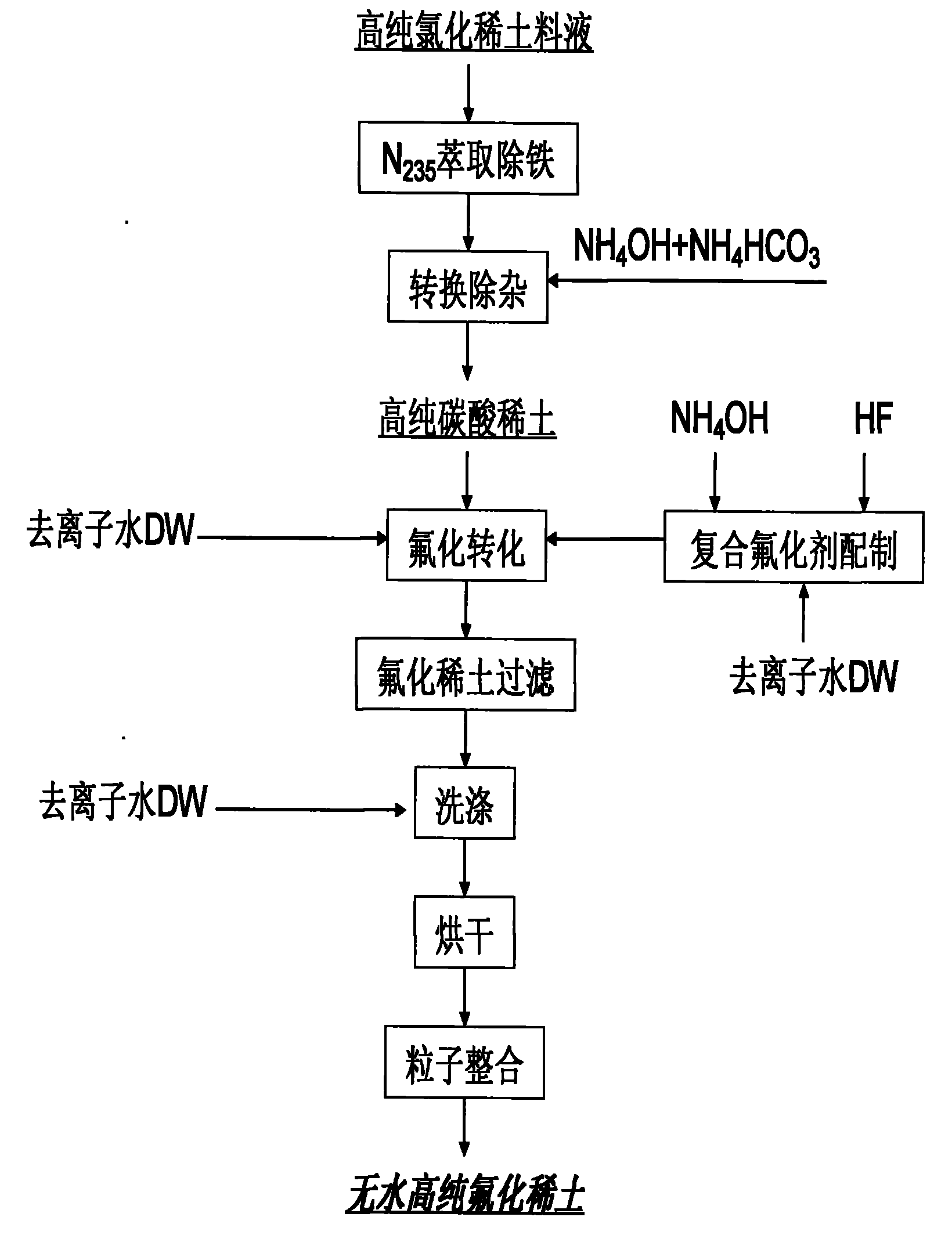
Image
Examples
Embodiment 1
[0023] Example 1 Anhydrous high-purity yttrium fluoride and its preparation
[0024] Preparation of high-purity yttrium chloride purification solution
[0025] REO concentration: 100-120g / l
[0026] Y purity: >99.999%
[0027] Fe 2 o 3 2 O<0.05mg / L
[0028] Free acidity: [H + ]≤0.50mol / L
[0029] Deionized water (DW)
[0030] Resistance > 12 megohms, total salinity 2 <70μg / l
[0031] Refined oxalic acid (H 2 C 2 O 4 ·2H 2 O)
[0032] Fe 2 o 3 2 <5ppm
[0033] Analytical grade hydrofluoric acid (HF)
[0034] Content (HF) > 40%
[0035] Fe 2 o 3 2 <5ppm
[0036] Chloride (Cl) <10ppm Phosphate <2ppm
[0037] Sulfates and sulfites (as SO 4 Count)<20ppm
[0038] 22% Reagent NH 4 Oh
[0039] Concentration (equivalent): 11.87mol / L
[0040] Fe 2 o 3 2 <5ppm
[0041] Ammonium bicarbonate solution
[0042] [OH - ] / [HCO 3 - ]=0.67-0.8
[0043] Fe 2 o 3 2 <5ppm
[0044] Compound fluorinating agent
[0045] Molar ratioHF / NH...
Embodiment 2
[0080] Example 2 Anhydrous high-purity lanthanum fluoride and its preparation
[0081] Preparation of Purification Solution of High Purity Lanthanum Chloride
[0082] REO concentration: 120-130g / l
[0083] La purity: >99.99%
[0084] Fe 2 o 3 2 O<0.05mg / L
[0085] Free acidity: [H + ]≤0.50mol / L
[0086] All the other conditions and operations are the same as in Example 1.
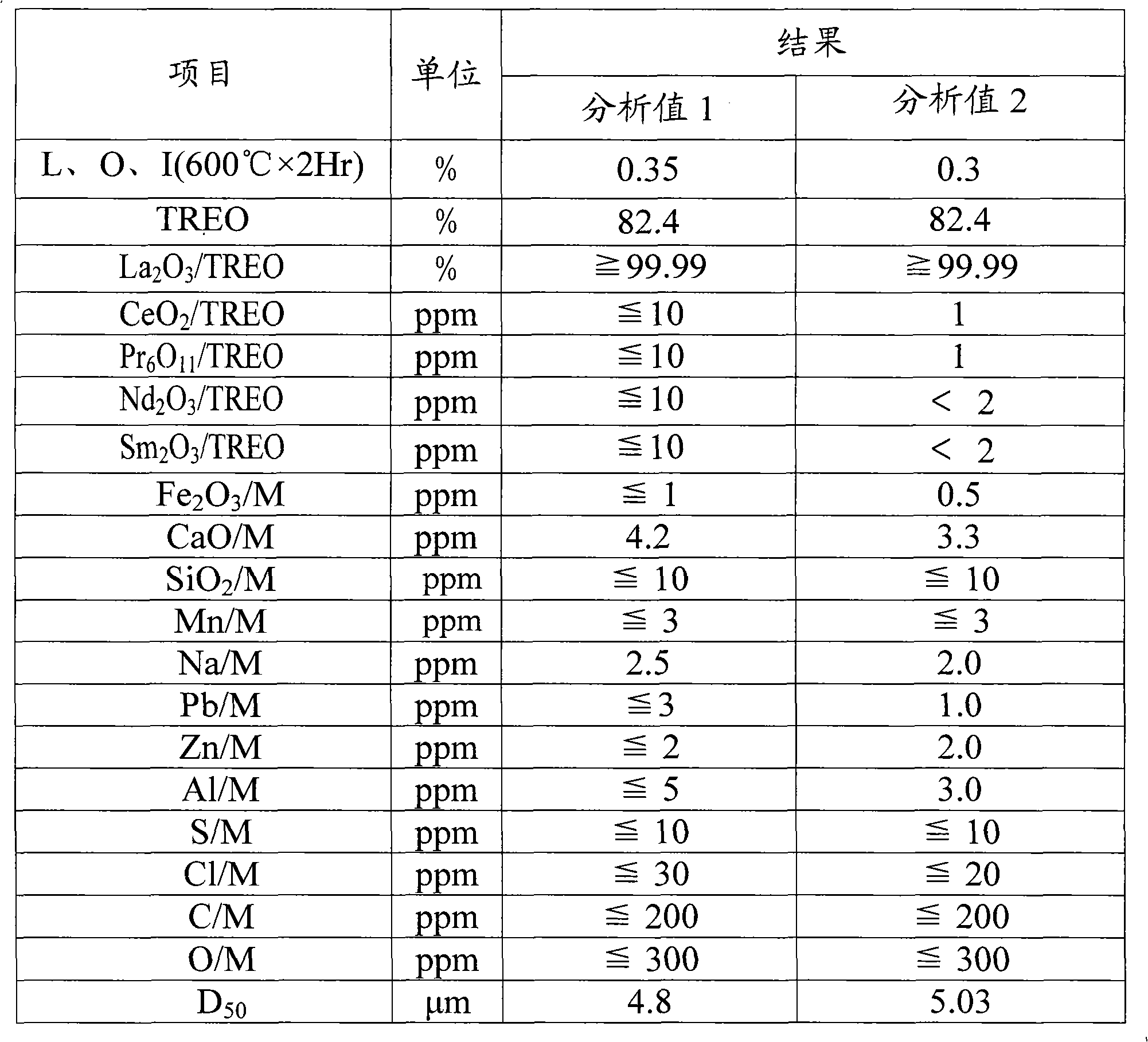
[0087] Anhydrous high-purity rare earth lanthanum fluoride quality index
[0088] project
unit
LaF 3
TREO
%
≥99.99
La 2 o 3 / TREO
ppm
≤10
CeO 2 / TREO
ppm
≤10
PR 6 o 11 / TREO
ppm
≤10
Nd 2 o 3 / TREO
ppm
≤10
SM 2 o 3 / TREO
ppm
≤10
Fe 2 o 3 / M
ppm
≤1
CaO / M
ppm
≤10
SiO 2 / M
ppm
≤10
Mn / M
ppm
≤1
Na / M
ppm
≤3
Pb / M
ppm
≤3
Zn / M
ppm
≤3
Al / M
ppm
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical resistance | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com