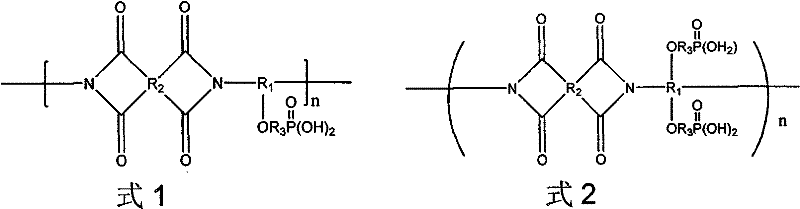Phosphate side chain-containing polyimide for gasoline desulphurization and preparation method thereof
A polyimide and side chain technology, which is applied in the field of polyimide and its preparation, can solve the problems that the octane number remains unchanged, does not involve changes in the content of aromatic hydrocarbons and octane number, etc. Simple preparation process and high desulfurization efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
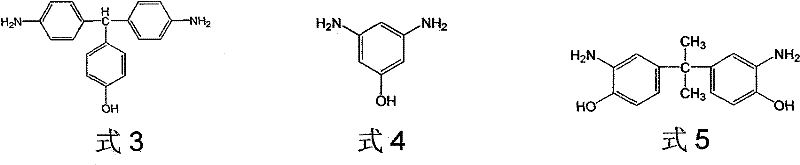
[0044] Under the protection of nitrogen, add 0.05mol of p-hydroxybenzaldehyde, 0.10mol of aniline, and 0.005mol of aniline hydrochloride into the reaction flask. After they are completely dissolved, react at 100°C for 1 hour, cool and precipitate the precipitate, and filter the precipitate to obtain the crude compound , after several times of recrystallization with methanol, evaporated to dryness to obtain pure 4,4-diamino, 4-hydroxytriphenylmethane (ie, compound a of formula 3).
[0045] Under nitrogen protection, dissolve 0.02mol 4,4-diamino,4-hydroxytriphenylmethane in 25ml N,N dimethylacetamide, add 0.02mol pyromellitic dianhydride, stir at room temperature for 3 hours, and then Add 12.5ml xylene solution, heat up to 100°C for dehydration imidization reaction for 5 hours to obtain compound b of formula 8 (wherein R 1 for R 2 for R 3 where n=0 means no hydrocarbon group)
[0046] Dissolve 0.004mol of compound b of formula 8 in 15ml of chloroform under nitrogen protec...
Embodiment 2
[0050] Under the protection of nitrogen, add 0.06mol p-hydroxybenzaldehyde, 0.48mol aniline solution, and 0.042mol aniline hydrochloride into the reaction flask. After they are completely dissolved, react at 150°C for 5 hours, cool and precipitate the precipitate, and filter the precipitate to obtain the crude product The compound was recrystallized several times with methanol, and evaporated to dryness to obtain pure 4,4-diamino, 4-hydroxytriphenylmethane (ie, compound a of formula 3).
[0051] Under nitrogen protection, 0.025mol 4,4'-diamino-4"-hydroxytriphenylmethane was dissolved in 40ml N,N dimethylformamide, and 0.030mol naphthalene tetraic acid dianhydride (ie, the compound of formula 16) was added, Stir at room temperature for 8 hours, then add 20ml of xylene solution, heat up to 130°C for dehydration imidization reaction for 10 hours, to obtain compound b of formula 8 (wherein R 1 for R 2 for R 3 where n=0 means no hydrocarbon group)
[0052] Dissolve 0.005 mol...
Embodiment 3
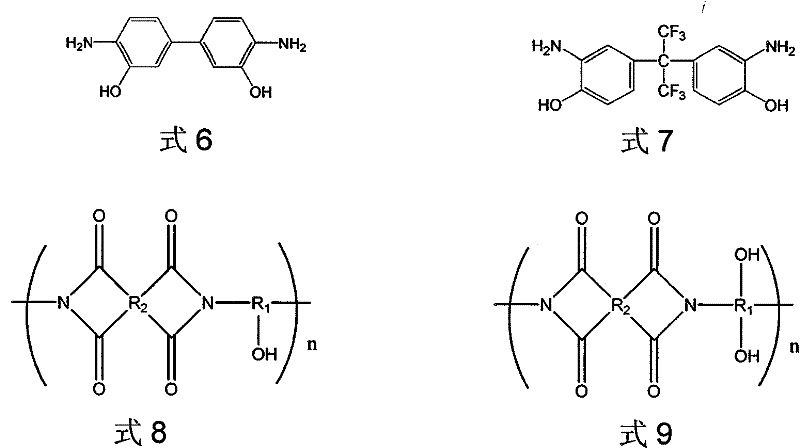
[0056] Under nitrogen protection, dissolve 0.02mol 3,3'-diamino-4,4'-dihydroxybiphenyl (compound a of formula 6) in 50ml N-methylpyrrolidone, add 0.026mol 3,3', 4,4'-diphenyl ether tetracarboxylic dianhydride (i.e. the compound of formula 18), stirred at room temperature for 8 hours, then added 25ml of xylene solution, raised the temperature to 150°C for dehydration imidization reaction for 12 hours, and obtained the compound of formula 9 Compound b (wherein R 1 for R 2 for R 3 where n=0 means no hydrocarbon group).
[0057] Dissolve 0.01 mol of compound b of formula 9 in 45 ml of dioxane under nitrogen protection, add 0.06 mol of triethylamine and 0.005 mol of cuprous chloride under ice-cooling, and then dropwise dissolve in 40 ml of dioxane within 20 minutes. Diethyl chlorophosphate in the six rings 0.05mol. React at room temperature for 12 hours, reclaim the filtrate, precipitate the polymer in methanol, and obtain the compound c1 of formula 11-1 after vacuum drying...
PUM
| Property | Measurement | Unit |
|---|---|---|
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com