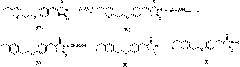Improved technological method for preparing pioglitazone hydrochloride
A technology for pioglitazone hydrochloride and process improvement, which is applied in the field of pioglitazone hydrochloride, a thiazolidinedione drug, can solve the problems of low yield of imine compounds, poor refining effect, and many reaction by-products, etc., and achieves easy and convenient post-treatment operation, hydrogenation The effect of high yield and improved purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
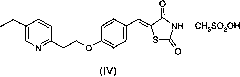
[0033] Preparation of (5-[[4-[2-(5-ethyl-2-pyridyl)ethoxy]phenyl]methylene]-2,4-thiazoledione mesylate (IV):
[0034] 27.2 g of 5-[[4-[2-(5-ethyl-2-pyridyl)ethoxy]phenyl]methylene]-2,4-thiazoledione (III) (HPLC purity: 96.1 %), methanesulfonic acid 7.74g and ethanol 136ml were added in the 250ml reaction flask, heated to reflux for 1 hour, cooled, filtered and dried to obtain 32.5g of white solid (IV).
[0035] Yield 95%, HPLC purity: 99.5%.
Embodiment 2
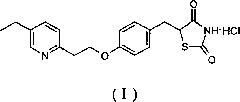
[0037] The preparation of pioglitazone hydrochloride (I):
[0038] Add 10g of the white solid (IV) obtained in Example 1, 50ml of glacial acetic acid, and 1.3g of 7% Pd / C (dry) into the hydrogenation kettle, empty the air, fill in hydrogen, and pressurize to 8-10kg / cm 2 , heated to 95 ° C under the condition of catalytic hydrogenation for 48 hours. The palladium carbon was removed by filtration, and the palladium carbon was washed with a small amount of glacial acetic acid, and the filtrates were combined and concentrated to obtain an oil. Add 100ml of water, stir to precipitate a white solid, and filter. The white solid obtained by filtration was adjusted to a pH value of about 7-9 with 25% concentrated ammonia water, filtered and dried to obtain 7.4 g of pioglitazone with a yield of 93.5%. Add 4 g of 30% ethanol hydrochloric acid solution and 105 ml of ethanol to the obtained pioglitazone, heat to reflux until completely dissolved, cool and precipitate to obtain a white so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com