Molecularly imprinted polymer and preparation method and application thereof
A molecular imprinting and polymer technology, applied in the field of material chemistry, can solve the problems of slow research progress and difficult dissolution of ractopamine, and achieve the effect of strong specific recognition ability, high affinity and selectivity, and improved specificity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
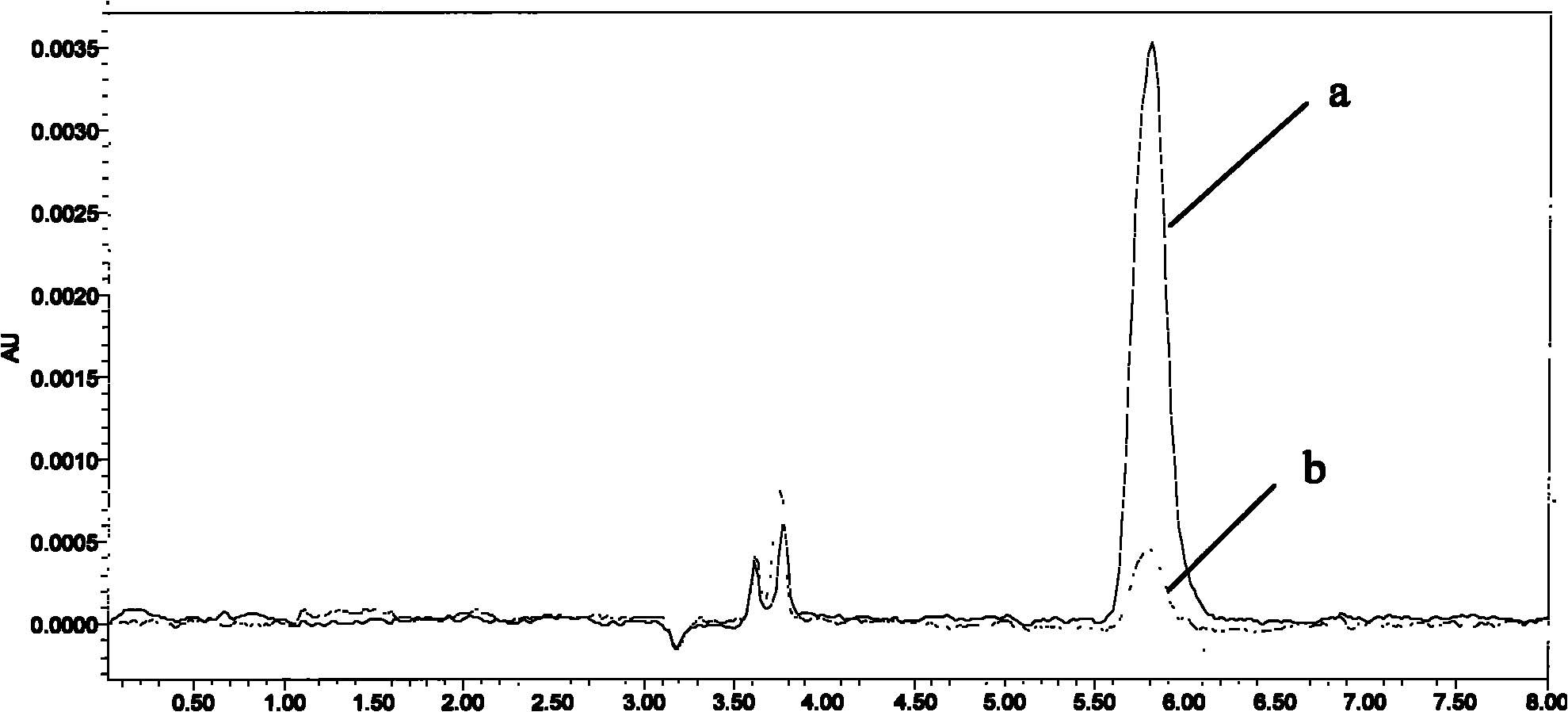
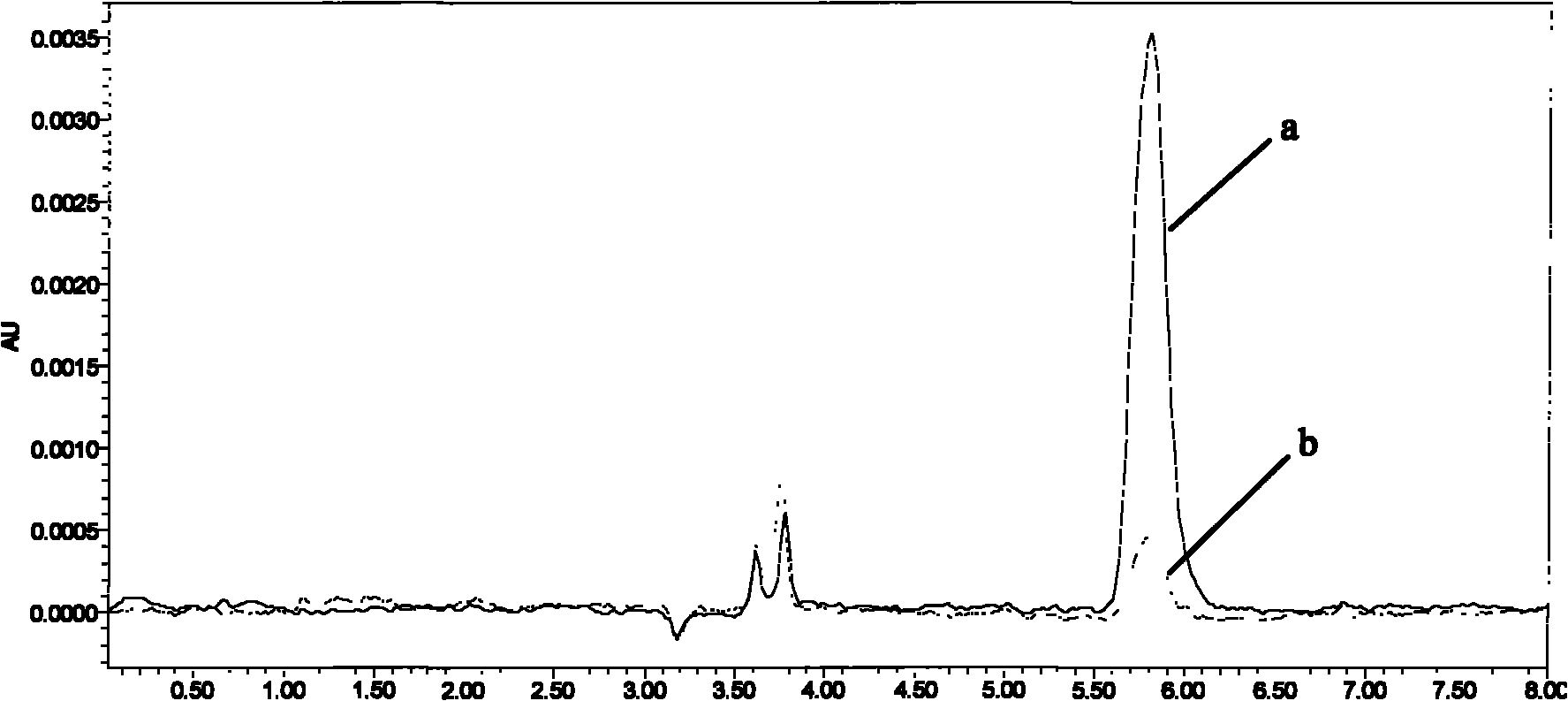
Image
Examples
Embodiment 1
[0036] Example 1 Molecularly imprinted polymer for detecting ractopamine
[0037] The synthesis and preparation steps of the molecularly imprinted polymer in this example are as follows:
[0038] (1) Preparation of prepolymer
[0039] Weigh 0.169g (0.5mmol) of ractopamine template into a test tube, add 3.0mL of acetonitrile and 0.1mL of triethylamine, after ultrasonic dissolution, add 142.2mg (2mmol) of acrylamide functional monomer, ultrasonic for 5min, and stand overnight at 4°C , forming a prepolymer.
[0040] (2) Polymerization curing
[0041] Add 1.89mL (10mmol) of ethylene glycol dimethacrylate and 15mg of azobisisobutyronitrile to the above prepolymer, sonicate for 5 minutes, blow nitrogen gas under ice bath for 2 minutes, seal it, and polymerize in a water bath at 60°C for 24 hours to obtain a block Polymer, the bulk polymer is the molecularly imprinted polymer used in the detection of ractopamine in this embodiment.
[0042] Grind the blocky polymer above, pass thro...
Embodiment 2
[0044] Example 2 Molecularly imprinted polymer for detecting ractopamine
[0045] The synthesis and preparation steps of the molecularly imprinted polymer in this example are as follows:
[0046] (1) Preparation of prepolymer
[0047] Weigh 0.169g (0.5mmol) of ractopamine template into a test tube, add 3.0mL of acetonitrile and 0.1mL of triethylamine, after ultrasonic dissolution, add 142.2mg (2mmol) of acrylamide functional monomer, ultrasonic for 5min, and stand overnight at 4°C , forming a prepolymer.
[0048] (2) Polymerization curing
[0049] Add 2.835mL (15mmol) of ethylene glycol dimethacrylate and 25mg of azobisisobutyronitrile to the above prepolymer, sonicate for 5 minutes, blow nitrogen gas under ice bath for 2 minutes, seal, and polymerize in a water bath at 60°C for 24 hours to obtain a block Polymer, the bulk polymer is the molecularly imprinted polymer used in the detection of ractopamine in this embodiment.
[0050] Grind the blocky polymer above, pass thro...
Embodiment 3
[0052] Example 3 Molecularly imprinted polymer for detecting ractopamine
[0053] The synthesis and preparation steps of the molecularly imprinted polymer in this example are as follows:
[0054] (1) Preparation of prepolymer
[0055] Weigh 0.169g (0.5mmol) of ractopamine template into a test tube, add 3.0mL of acetonitrile and 0.1mL of triethylamine, after ultrasonic dissolution, add 71.08mg (1mmol) of acrylamide functional monomer, ultrasonic for 5min, and stand overnight at 4°C , forming a prepolymer.
[0056] (2) Polymerization curing
[0057] Add 1.89 mL (10 mmol) of ethylene glycol dimethacrylate and 25 mg of azobisisobutyronitrile to the above prepolymer, sonicate for 5 minutes, blow nitrogen gas under ice bath for 2 minutes, seal, and polymerize in a water bath at 60°C for 24 hours to obtain a block Polymer, the bulk polymer is the molecularly imprinted polymer used in the detection of ractopamine in this embodiment.
[0058] Grind the blocky polymer above, pass th...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com