P53 protein monoclonal antibody and application thereof
A monoclonal antibody and protein technology, applied in the field of biological sciences, can solve the problems of difficult antibody preparation and screening methods, and the detection effect of different tumor samples needs to be improved, so as to achieve the effect of strong specific recognition effect and strong positive signal
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
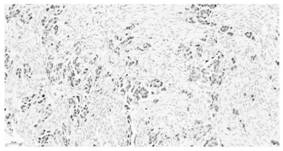
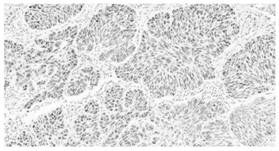
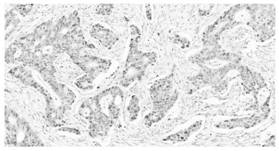
Image
Examples
Embodiment 1
[0038] The preparation of embodiment 1 immunogen
[0039] 1. Construction of p53 recombinant expression vector
[0040] By means of conventional gene synthesis, according to the amino acid composition, antigenicity, hydrophilicity and hydrophobicity of the sequence, the secondary structure and the specificity of the sequence, etc., the 352nd to 352nd to the p53 protein encoding the amino acid sequence shown in SEQ ID NO: 7 were selected. The gene sequences of amino acids 366, 1-131, 99-112, 295-376, 1-80 and 15-39 were inserted into the E.coil pet32a vector by restriction endonucleases EcoRI and xhoI, respectively. Construct the recombinant expression plasmid of p53. The amino acid sequence of human p53 protein was obtained from the Uniprot database (https: / / www.uniprot.org / uniprot / P04637#sequences).
[0041] 2. Expression and purification of p53 recombinant protein
[0042] The p53 recombinant expression plasmid constructed in step 1 was transformed into Escherichia coli R...
Embodiment 2
[0043] The preparation of embodiment 2 polypeptide
[0044] The method of chemical synthesis synthesizes polypeptides with amino acid sequences as shown in SEQ ID NO: 1-6, and synthesizes one more cysteine (Cyc) at the N-terminus of the polypeptides to obtain polypeptides 1 to 6 for coating enzyme labels Version.
Embodiment 3
[0045] Embodiment 3 immune mice and carry out ELISA screening
[0046] The p53 recombinant protein 5 purified in Example 1 (amino acid sequence shown in SEQ ID NO: 5) was used to immunize female balb / c mice aged 6 to 8 weeks, with an interval of 3 weeks between the first immunization and booster immunization, and subsequent booster immunization The interval was 2 weeks, at least 4 times of immunization, the spleen was taken to make a single cell suspension, and the SP2 / 0 cells in the logarithmic growth phase were taken at the same time 1×10 7 One, using the commercial reagent PEG1450 (Sigma, P7181) for cell fusion, uniformly dilute the fused hybridoma cells into a certain volume of 1% HAT (Sigma, H0262) selection medium, and plate 96-well cell culture plates for 6 ~8 pieces, 200 μL / well, after static culture for 4 to 6 days, visible hybridoma cell colonies are formed, and the liquid is changed to obtain the supernatant on the fusion plate, which is to be tested.
[0047] Use ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com