Method for detecting nitromidazole residue in royal jelly by using high performance liquid chromatography tandem mass spectrum
A technology of high performance liquid chromatography and nitroimidazoles, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of difficult sample purification, increased detection cost, and less reagent consumption, and achieves low ion suppression effect and increased The amount of solvent used and the effect of reducing the amount of solvent used
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
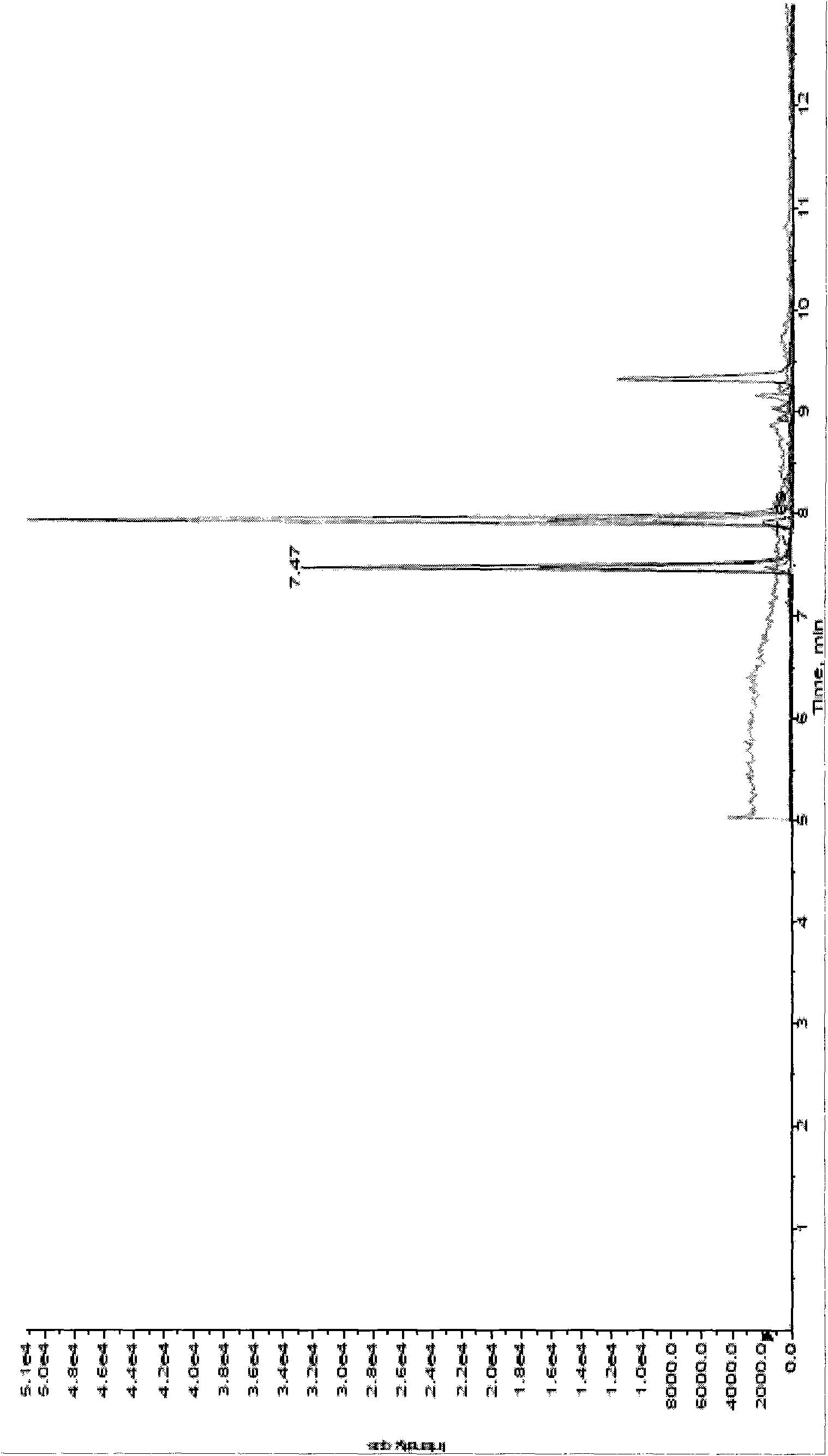
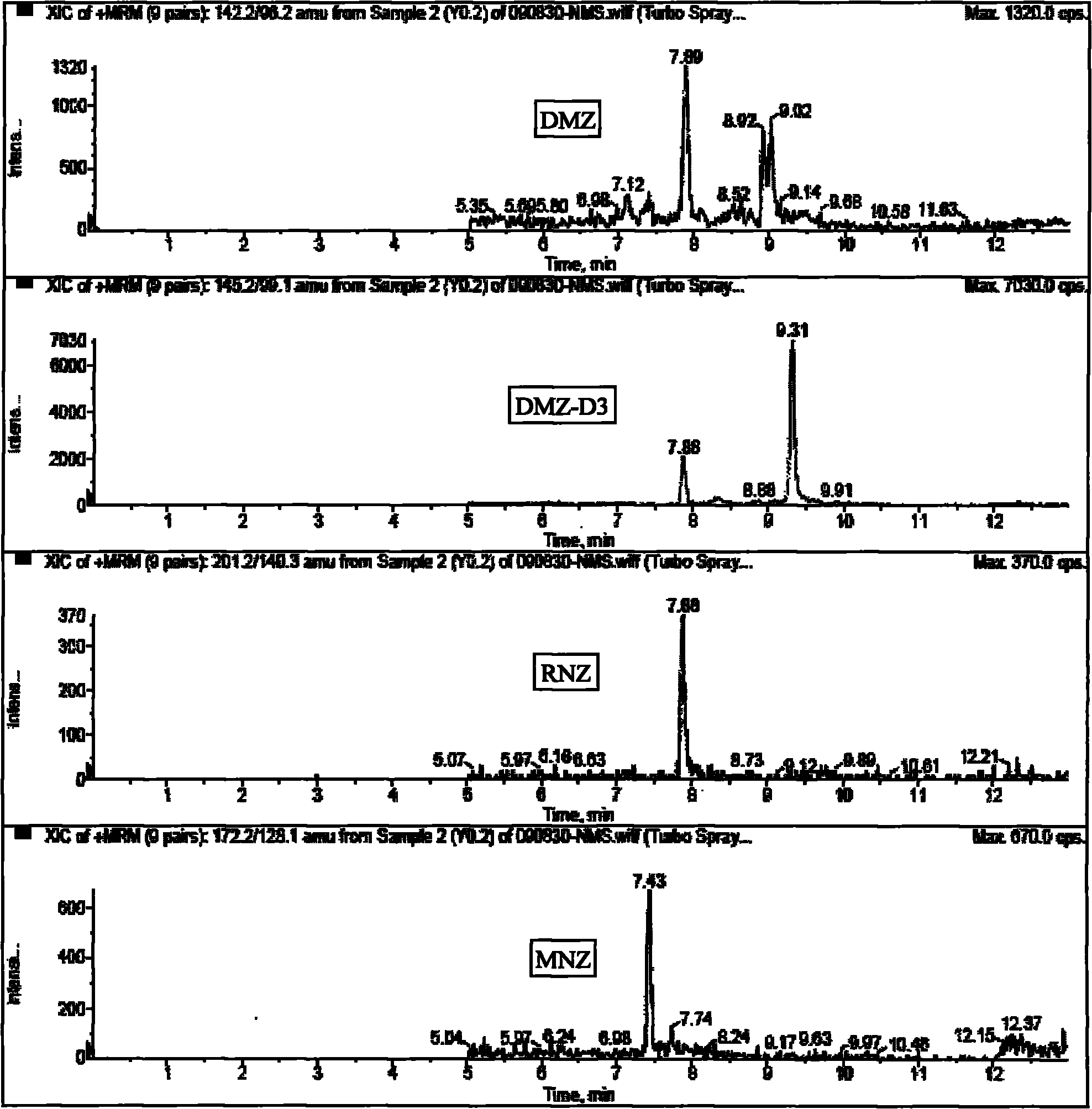
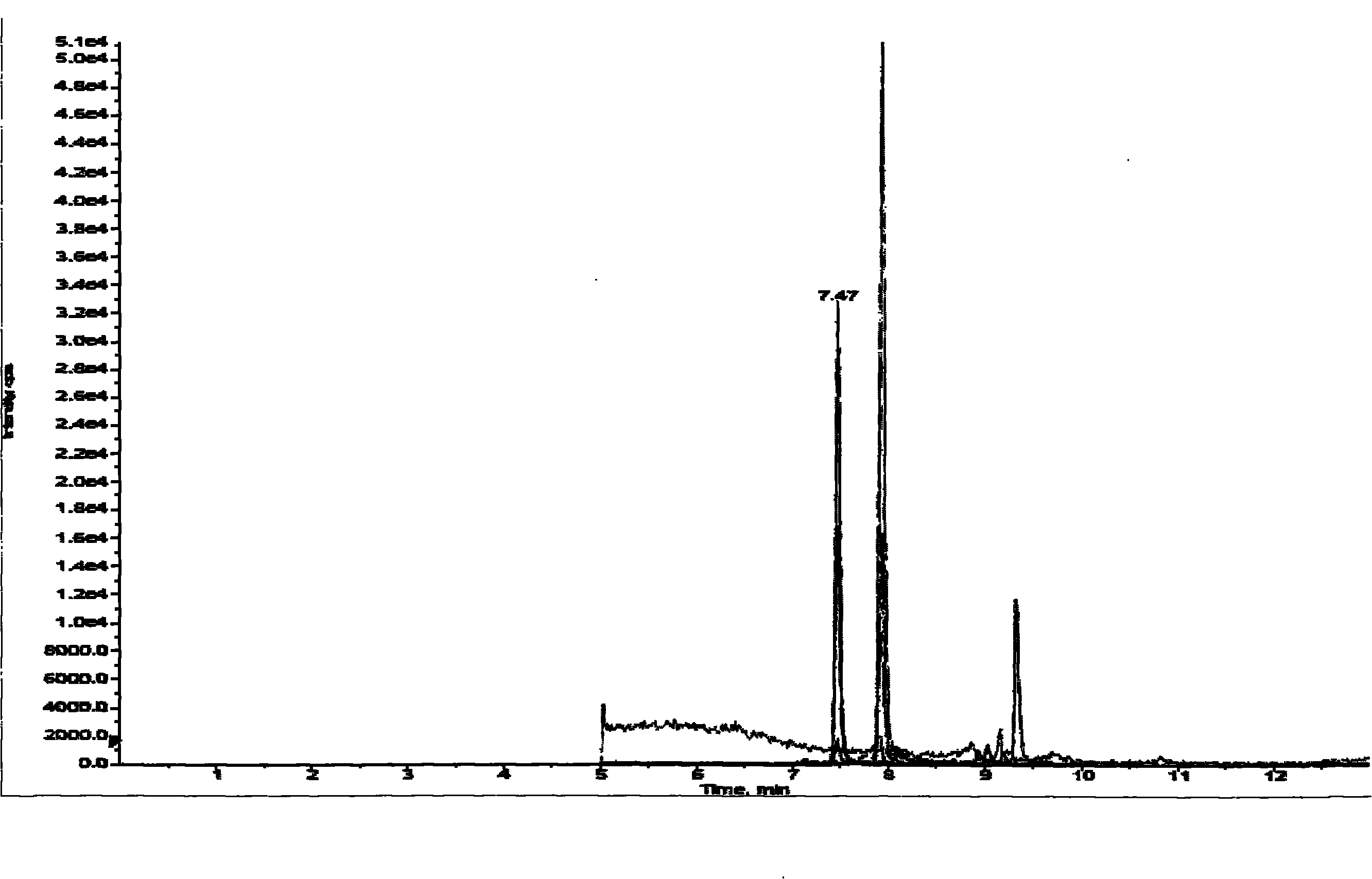
[0024] see figure 1 and figure 2 The method for measuring nitroimidazole residues in royal jelly by high performance liquid chromatography tandem mass spectrometry in this embodiment includes a standard solution preparation process, a royal jelly sample processing process, a standard curve preparation process and a royal jelly sample detection process.
[0025] The raw materials used in this example include metronidazole, Metronidazole, CAS: [443-48-1], purity > 99.4%; ronidazole, ronidazole, CAS: [7681-76-7], purity > 99.0% %; Dimetridazole, dimetridazole, CAS: [551-92-8], purity > 98.5%; D3-Dimetridazole-d3, CAS: 64678-69-9, purity > 98%; Residual grade of ethyl acetate; analytically pure sodium hydroxide and sodium chloride; superior grade of n-hexane; liquid chromatography pure acetonitrile, ammonium acetate and formic acid; water; initial mobile phase. Wherein metronidazole, ronidazole, dimetidazole and D3-dimetridazole all come from Dr.Ehrenstorfer company of Germany;...
PUM
| Property | Measurement | Unit |
|---|---|---|
| correlation coefficient | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com