Polyclone antibody of H-9201, and preparation method and application thereof
A technology of H-9201 and metolaphos, which is applied in chemical instruments and methods, compounds of Group 5/15 elements of the periodic table, and material testing products, etc., to achieve the effect of a simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] Example 1. Preparation of Amisofos Transition State Similar Compounds
[0038] Phosphor trichloride and methanol are used as raw materials, the temperature of the solution is controlled below -5°C for reaction, the solution is washed with ice water and the lower layer is retained to obtain methoxyphosphoryl thiodichloride.
[0039] Under the temperature-controlled condition of acetone solution at 5-10°C, triethylamine is used as a catalyst, methoxyphosphoryl thiol) and 2,4-dimethyl-6-nitrophenol (DMNT), sodium hydroxide (NaOH ) reaction to obtain an acetone solution containing the compound of molecular structural formula (5).
[0040] The compound solution containing the molecular structural formula (5) is pre-cooled to 5-10°C, and ammonia water is added dropwise thereto. After the ammonia water is added dropwise, the pH is about 8, add 40% aqueous sodium hydroxide solution dropwise until the pH is 10, remove the acetone, add 30 mL of diethyl ether to extract twice, wa...
Embodiment 2
[0042] Embodiment 2. Preparation of the compound of molecular structural formula (3)
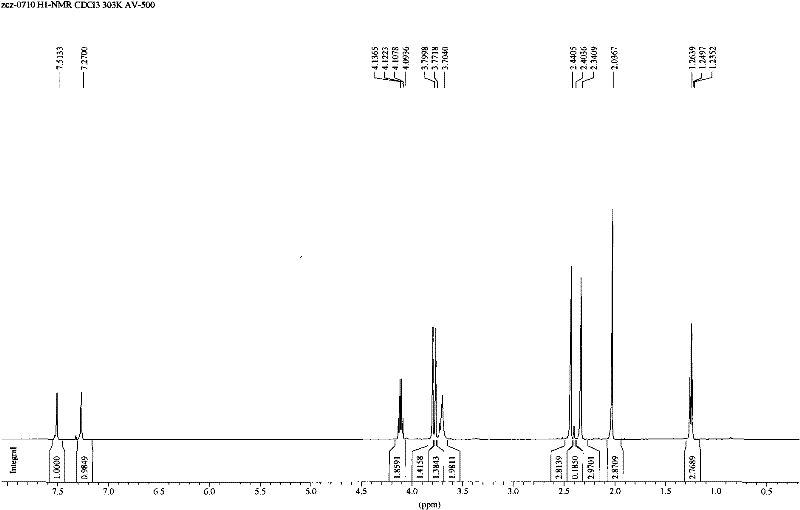
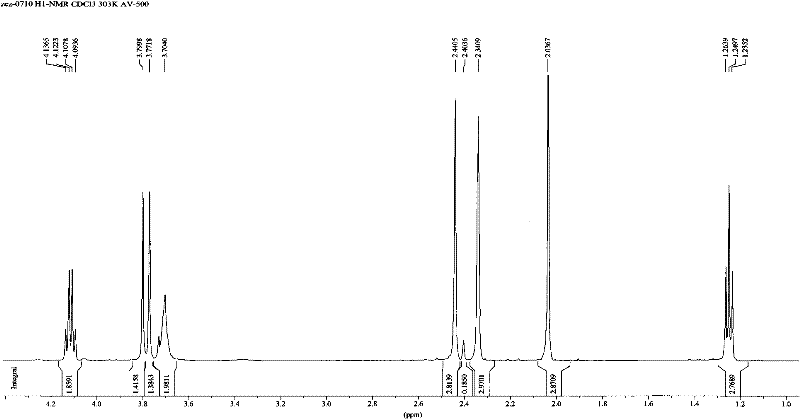
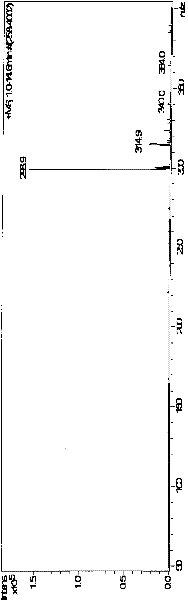
[0043] Mix 10ml of 0.5mM dimethazone transition state analogue compound with 0.8mM succinic anhydride acetonitrile solution, add dropwise 0.5mM DMAP acetonitrile solution, and stir for 2 hours at 50°C; after the reaction is terminated, add 50ml of distilled water, Extraction with methyl chloride, dehydration of the organic phase with anhydrous magnesium sulfate, and removal of the organic solvent to obtain the compound of molecular structure formula (3). Its liquid chromatography / mass spectrometry identification chart (LC / MS) see Figure 4 .
Embodiment 3
[0044] Example 3. Synthesis of antigens
[0045] Add 0.23mM NHS, 0.33mM DCC, 0.033mM DMAP to the dichloromethane solution containing 0.3mM compound of molecular formula (3), stir and react overnight at 0°C; centrifuge at 10000g for 10min the next day, discard the precipitate, and remove Solvent, dissolve the obtained activated ester with DMF, slowly add the activated ester solution to 5 mL of pre-cooled phosphate buffer containing 40 mg bovine serum albumin BSA, the pH of the phosphate buffer is 9.0, and react overnight at 4°C;
[0046] After the reaction, put the reaction mixture into a dialysis bag, dialyze in deionized water at 4°C for 3 days, change the water every 8 hours, obtain the immune antigen with molecular structure formula (1) after the dialysis, subpackage and store in - Store in the refrigerator at 20°C for later use. Such as Figure 5 Figure 6 The UV scanning spectrum shown shows that the maximum absorption peaks of hapten (hapten) and carrier protein (BSA ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com