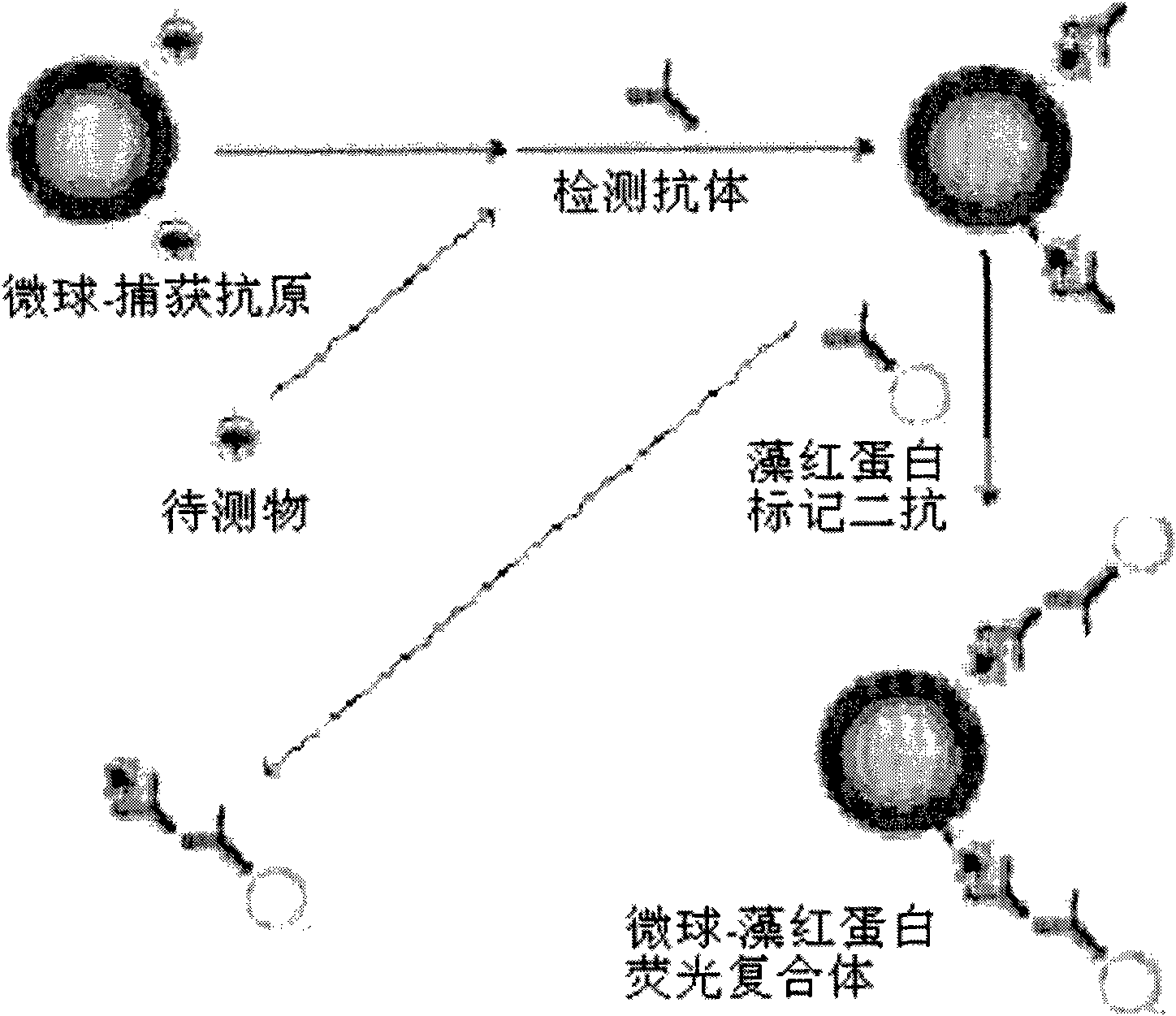
Synchronous and indirect competitive immunological detection method and kit of plural small molecular compounds
A technology for simultaneous detection of small molecular compounds, applied in measurement devices, analytical materials, material excitation analysis, etc., to achieve the effect of large flux and high sensitivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
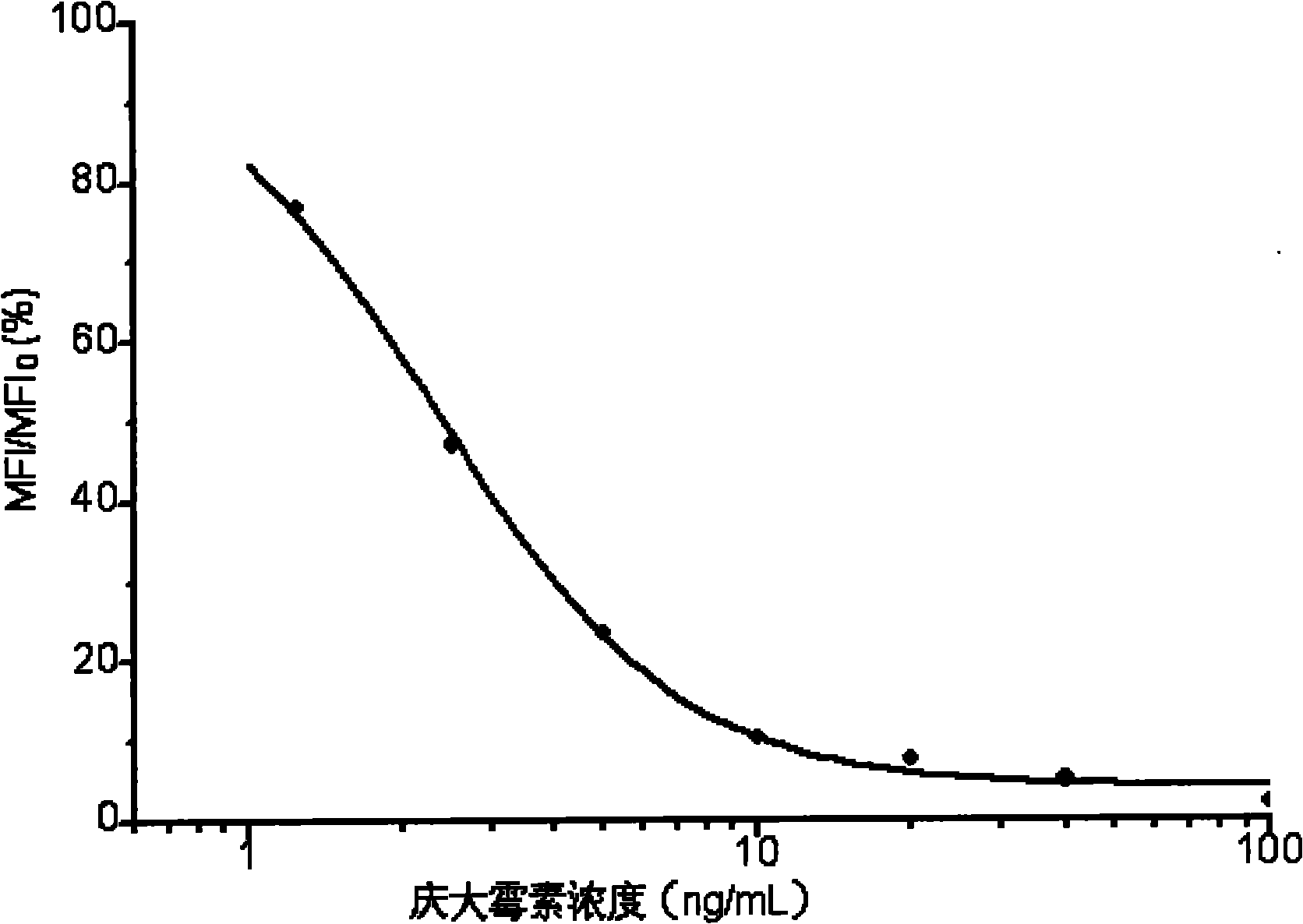
[0037] The microsphere indirect competitive immunofluorescence detection of gentamicin includes the following specific steps:
[0038] 1. Microsphere-capture antigen coupling:
[0039] Take 1.25×10 6 Each microsphere is a batch. In a 1.5mL centrifuge tube, use EDC and sulfo-NHS solution to activate the carboxyl groups on the surface of the microspheres at pH 6.2 for 20 minutes at room temperature, and then replace the microspheres with phosphate buffer ( In PBS, 0.01M, PH7.4), add an optimized amount of gentamicin-BSA-coupled capture antigen, shake for 2 hours at room temperature in the dark, centrifuge to wash off unbound capture antigen, and wash with 1% BSA Resuspend in PBS, avoid light and shake at room temperature for 0.5 hours to block the remaining sites on the surface of the microspheres that are not bound to the capture antigen. Finally, store the microsphere-capture antigen conjugate in PBS containing 0.1% BSA.
[0040] 2. Phycoerythrin-secondary antibody labeling:...
Embodiment 2
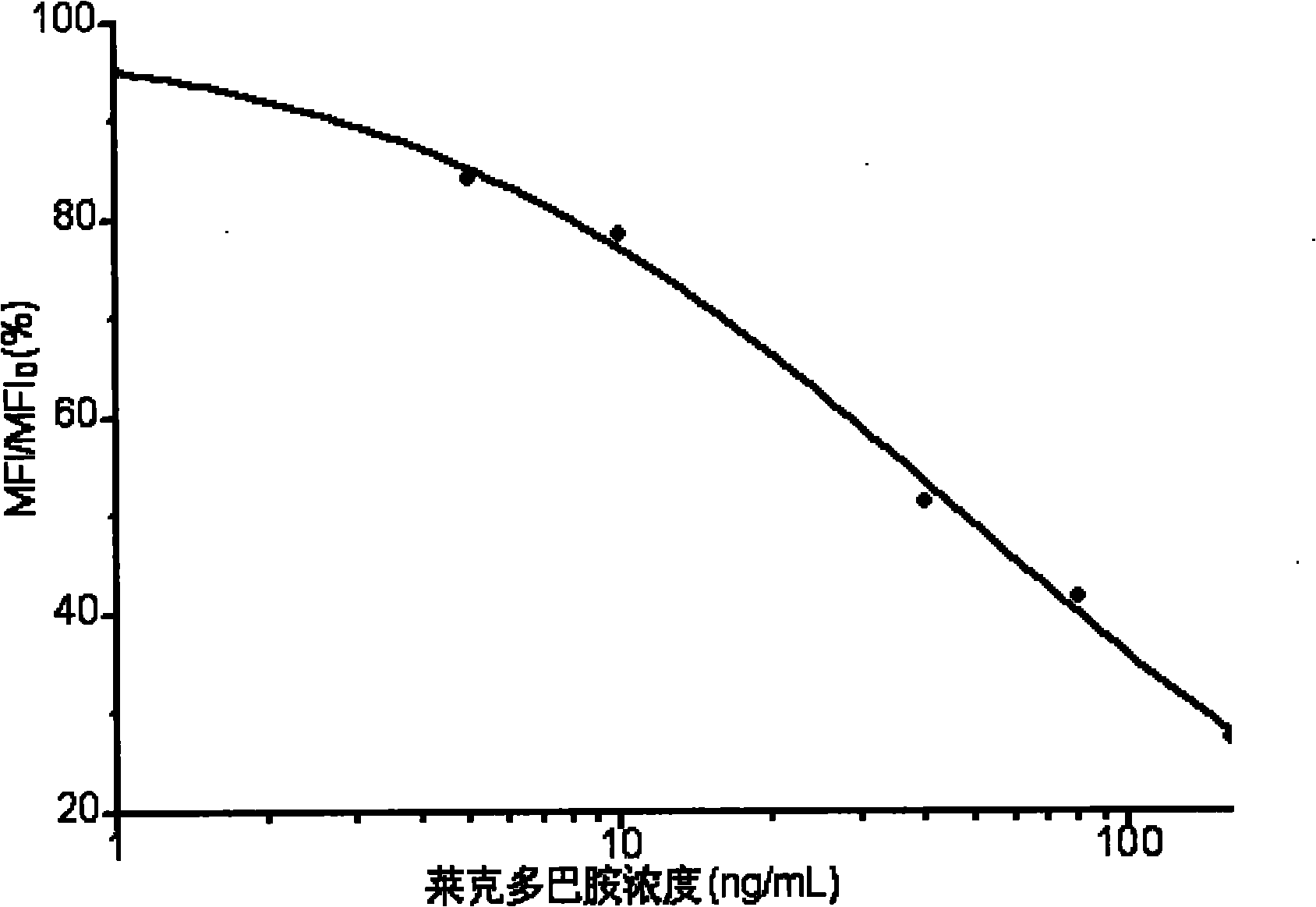
[0047] The microsphere indirect competitive immunofluorescence detection of ractopamine includes the following specific steps:
[0048] The operations were the same as those in Example 1, except that the capture antigen and detection antibody were ractopamine-BSA conjugate and anti-ractopamine monoclonal antibody, and the small molecule antigen to be detected was ractopamine. Similarly, the sample to be tested containing ractopamine is added to the reaction system, and the concentration of ractopamine in the sample is obtained by comparing the fluorescence intensity with the standard curve. Taking the logarithm of the concentration of ractopamine as the abscissa, MFI / MFI 0 The ratio of is the ordinate, draw the standard curve, see image 3 .
Embodiment 3
[0050] The microsphere indirect competitive immunofluorescence detection of simultaneous detection of kanamycin and gentamicin includes the following specific steps:
[0051] 1. Microsphere-capture antigen coupling:
[0052] Take the microspheres coded as 20 and 30 respectively 1.25×10 6 Each is a batch, and in two 1.5mL centrifuge tubes, use EDC, sulfo-NHS solution to activate the carboxyl groups on the surface of the microspheres at room temperature for 20 minutes under the condition of pH6.2, and then replace the microspheres with phosphate buffer ( In PBS, 0.01M, pH7.4), add the optimized amount of kanamycin-BSA and gentamycin-BSA-coupled capture antigen respectively, shake and react at room temperature for 2 hours in the dark, and centrifuge to wash off the unbound antigen. Capture the antigen, resuspend it with PBS containing 1% BSA, and shake it in the dark to react at room temperature for 0.5 hours. Block the remaining sites on the surface of the microspheres that are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com