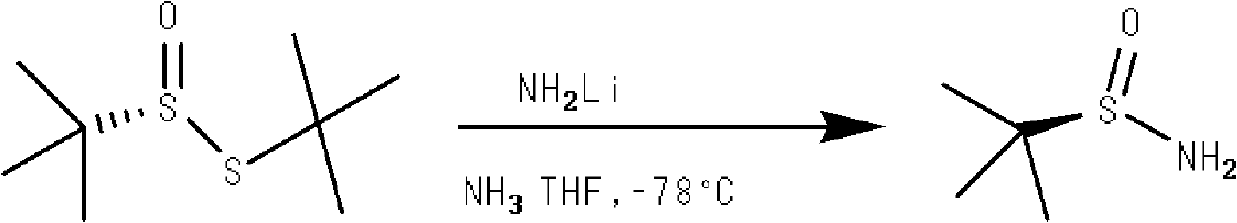Process method for synthetizing tert-butyl sulfinamide
A technology of tert-butylsulfinamide and process method, which is applied in the field of synthesis of organic compounds, and achieves the effects of good process stability, reduced production cost, and easy procurement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] A kind of technical method of synthesizing tert-butyl sulfinamide, synthetic route is as follows:
[0029] The specific steps are:
[0030] Example 1
[0031] a) Synthesis of N-methyltriphenylamine: under argon protection, add triphenylchloromethane (0.1076 mol) to a 1-liter reaction flask equipped with mechanical stirring and cooling bath, and maintain the temperature at -50 Between -60°C, slowly feed 600mL of liquid ammonia, add toluene with 1% triphenylchloromethane mass as internal standard analysis, triphenylchloromethane internal standard analysis is less than 1%, and the reaction stops after 4-6 hours. The reaction solution was raised to room temperature, and the ammonia gas was volatilized. Add 500mL methyl tert-butyl ether (MTBE), wash the organic layer with 70g mass concentration of 10% sodium carbonate, wash the organic layer with 75g*2 water, dry and concentrate to obtain N-methyl Triphenylamine crude product 24g. 1.5*24g diethyl ether beating, filter a...
Embodiment 2
[0036] A kind of processing method of synthesizing tert-butylsulfinamide, reaction formula is as embodiment 1, and concrete steps are:
[0037] a) Synthesis of N-methyltriphenylamine: under the protection of argon, add triphenylchloromethane (0.1076 mol) to a 1-liter reaction flask equipped with mechanical stirring and cold bath, and maintain the temperature at -60 Between ~-70°C, slowly inject 600mL of liquid ammonia, add toluene with 1% mass of triphenylchloromethane as the internal standard analysis, the internal standard analysis of triphenylchloromethane is less than 1%, and the reaction stops after 4-6 hours. The reaction solution was raised to room temperature, and the ammonia gas was volatilized. Add 500mL ethyl acetate, wash the organic layer with 70g mass concentration of 10% sodium carbonate, wash the organic layer with 75g*2 water, dry and concentrate to obtain the crude product of N-methyltriphenylammonia 25.5 g. 1.5*25.5g n-hexane beating, filter after 1.5h, and...
Embodiment 3
[0042] A kind of processing method of synthesizing tert-butylsulfinamide, reaction formula is as embodiment 1, and concrete steps are:
[0043] a) Synthesis of N-methyltriphenylammonia: under the protection of argon, add triphenylbromethane (0.1076 mol) to a 1-liter reaction flask equipped with mechanical stirring and cold bath, and maintain the temperature at -60~ Between -70°C, slowly feed 600mL of liquid ammonia, add toluene with 1% mass of triphenylbromomethane as internal standard analysis, triphenylbromomethane internal standard analysis is less than 1%, and the reaction stops after 4-6 hours. The reaction solution was raised to room temperature, and the ammonia gas was evaporated. Add 500mL MTBE, wash the organic layer with 70g mass concentration of 10% sodium carbonate, wash the organic layer with 75g*2 water, dry and concentrate to obtain 26g of crude N-methyltriphenylamine. 1.5*26g of diethyl ether was beaten, filtered after 1.5h, and the dried product was 0.0930mol....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com