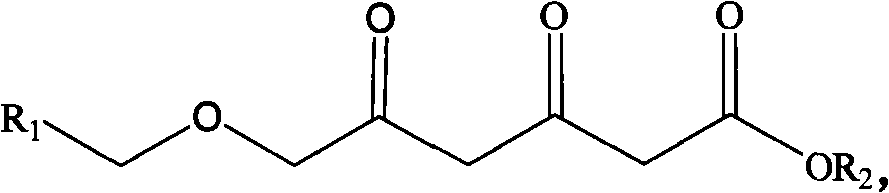
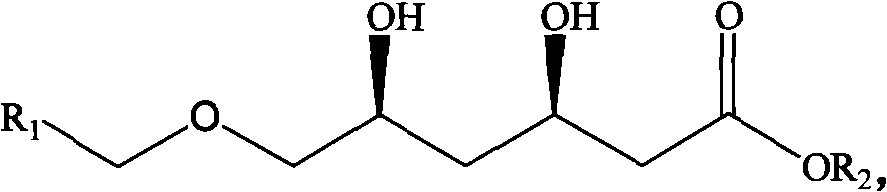
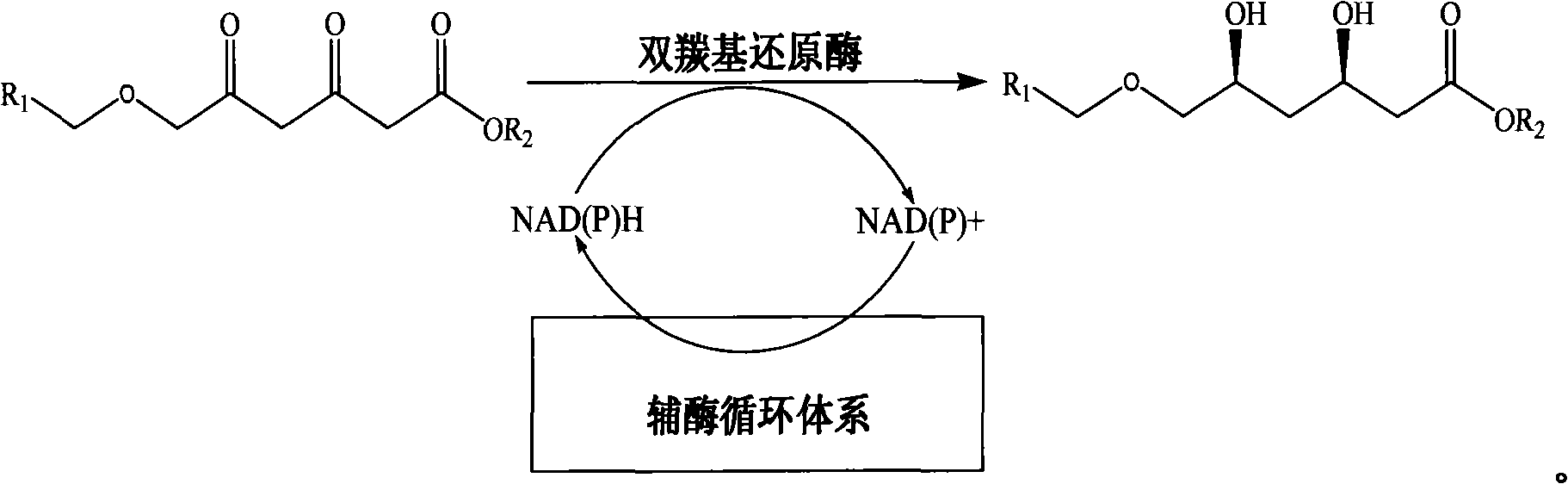
Method for preparing chiral 3R, 5S-dihydroxyl compound by nonaqueous phase
A technology for dihydroxy compounds and chiral compounds is applied in the field of asymmetric preparation of chiral pharmaceutical intermediates by biocatalysis, and achieves the effects of high efficiency, good application prospects and simple preparation methods.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A method for preparing 3R, 5S-dihydroxy-6-benzyloxy-ethylhexanoate, comprising the following steps: in a 1ml reaction system, sequentially add 9.5-140mg of sodium formate, 3,5-dicarbonyl-6- Benzyloxy-ethyl hexanoate 10~150mg, 0.1M (pH=6.0) potassium phosphate buffer, NAD + 20μl (0.5mM), formate dehydrogenase 27.2mg (4U / ml), double carbonyl reductase supernatant 65μl (6U / ml), and finally add n-hexane 500μl; react at room temperature and shaking speed of 200rpm for 18 hours, After the reaction was stopped, the samples were separated and purified, and the samples were subjected to high-performance liquid chromatography to analyze the conversion rate of the substrate and the optical purity of the product.
[0037] When the substrate concentration is less than or equal to 100g / L, the conversion rate is greater than 99%, and the conversion rate at 125g / L is 95.1%, and the ee and de of the product 3R, 5S-dihydroxy-6-benzyloxy-ethyl hexanoate The values are all higher than 9...
Embodiment 2
[0042] A method for preparing 3R, 5S-dihydroxy-6-benzyloxy-ethylhexanoate, comprising the steps of: adding 9.5 mg of sodium formate and 3,5-dicarbonyl-6-benzyl to a reaction system of 1 ml successively Oxy-ethylhexanoate 10mg, 0.1M (pH=6.0) potassium phosphate buffer, NAD + 20μl (0.5mM), formate dehydrogenase 27.2mg (4U / ml), double carbonyl reductase supernatant 65μl (6U / ml), and finally add n-hexane 500μl; react at 40°C and shaking speed 200rpm for 18 hours After the reaction is stopped, separation and purification are carried out, and the sample is subjected to high performance liquid chromatography to analyze the conversion rate of the substrate and the optical purity of the product.
[0043] The substrate conversion rate was 76.4%, and the ee and de values of the product 3R, 5S-dihydroxy-6-benzyloxy-ethylhexanoate were both higher than 99.5%.
Embodiment 3
[0045] A method for preparing 3R, 5S-dihydroxy-6-benzyloxy-ethyl hexanoate, comprising the steps of: in a reaction system of 1ml, adding sodium formate 140mg (2M), 3,5-dicarbonyl- 6-Benzyloxy-ethylhexanoate 10g / L, 0.1M (pH=6.0) potassium phosphate buffer, NAD + 20μl (0.5mM), formate dehydrogenase 27.2mg (4U / ml), double carbonyl reductase supernatant 65μl (6U / ml), finally add ethanol 100-400μl, react at room temperature and shaking speed 200rpm for 18 hours , after the reaction was stopped, separation and purification were carried out, and the sample was analyzed by high performance liquid chromatography for substrate conversion rate and product optical purity.
[0046] When the ethanol content is 20% (v / v), the conversion rate is 95.0%, and the conversion rate is 3.3% at 30%, the ee and de values of the product 3R, 5S-dihydroxy-6-benzyloxy-ethyl hexanoate Both are higher than 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com