C4 hydrocarbon catalysis and separation method capable of separating isobutene and butene-2
A technology for isobutene and C4-hydrocarbons, which is applied in the field of C4-hydrocarbons catalytic separation of isobutene and butene-2, which can solve the problems of low isomerization rate of butene-1 double bond, low purity of isobutene, complicated separation methods, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
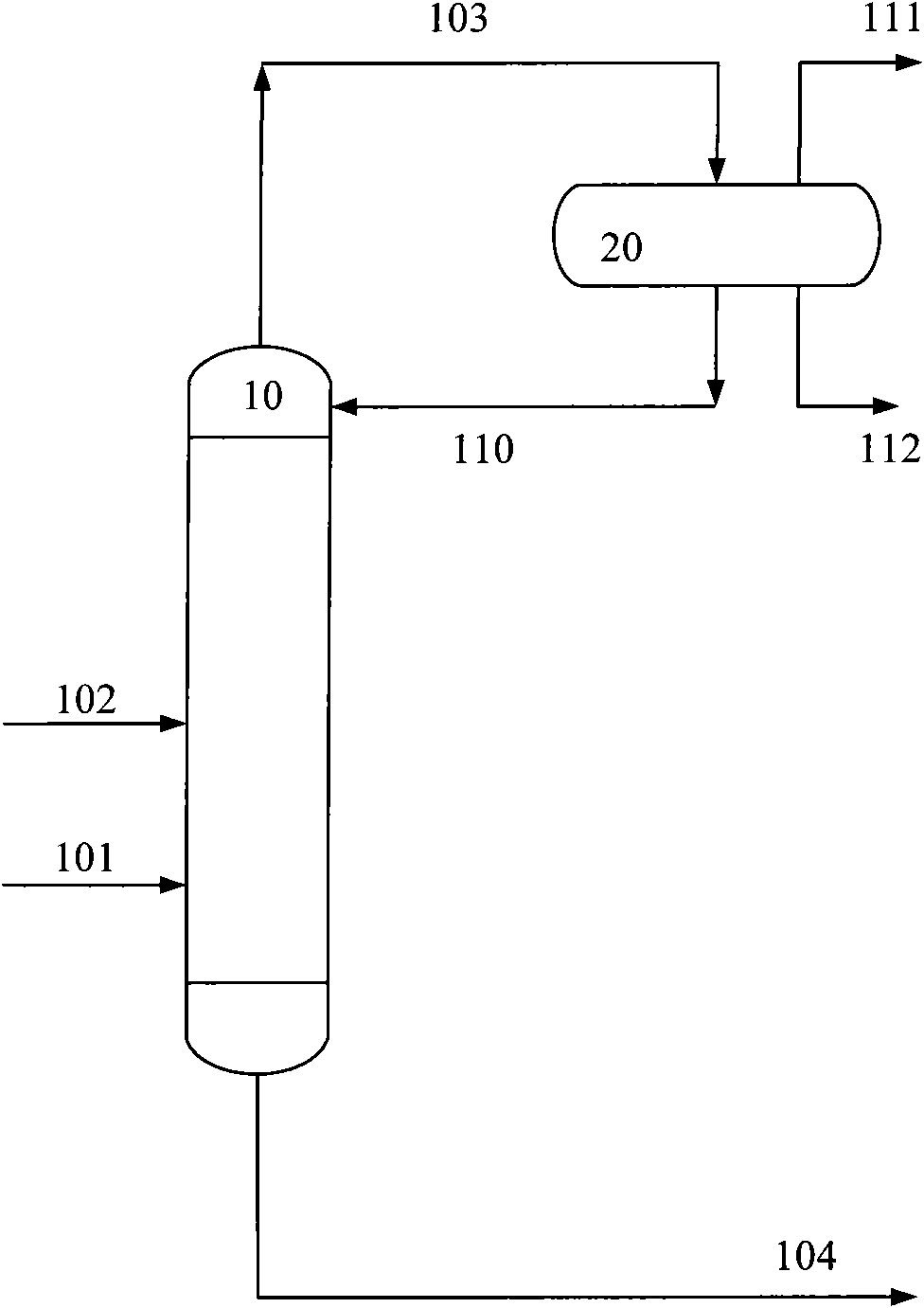
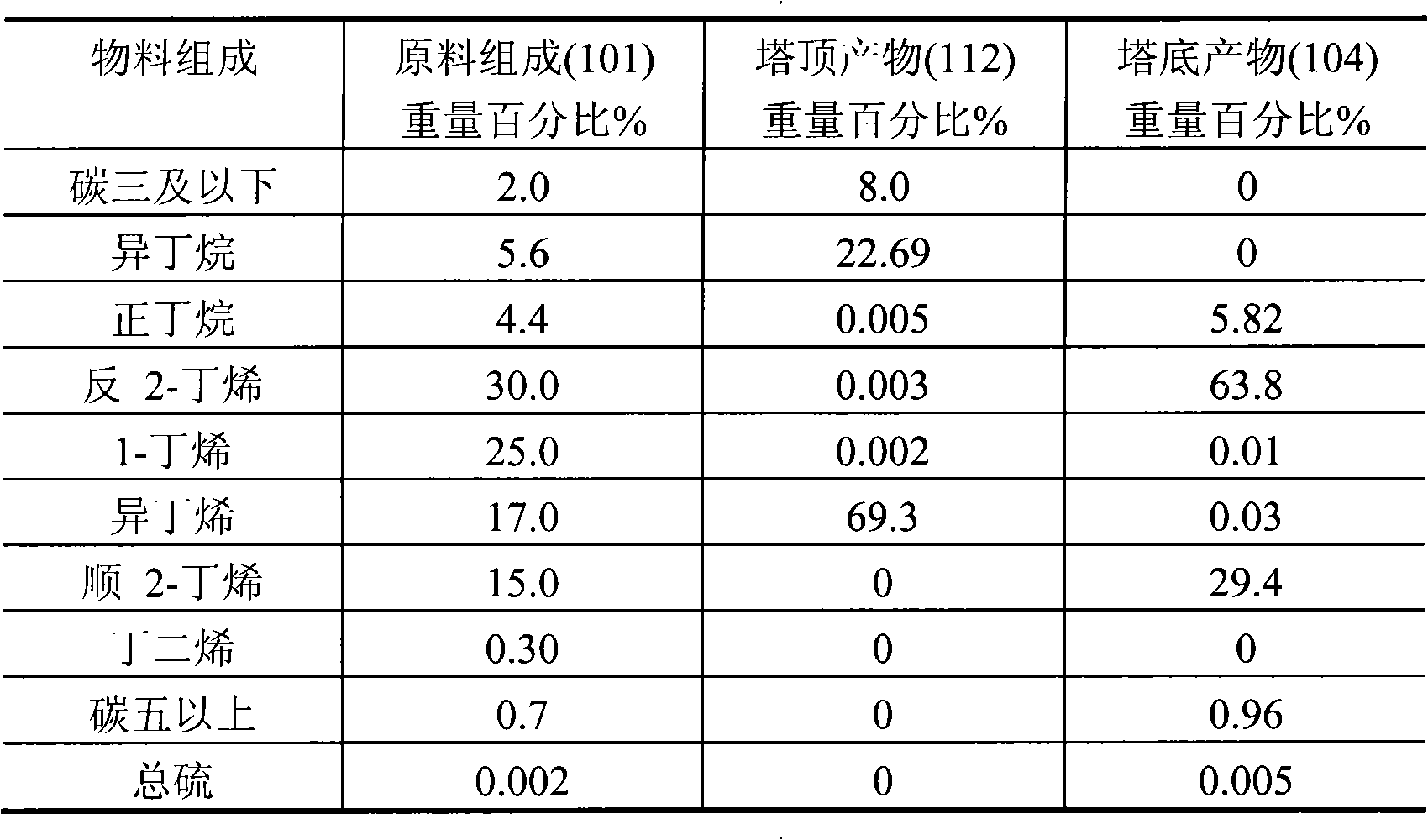
[0015]A reactive distillation column with a diameter of 10 cm and a height of 20 meters has a total of 100 trays from the bottom to the top of the column, numbered 1, 2, 3, ... 100 in sequence. Two layers of catalysts are filled in the tower, and the catalyst of the lower layer is loaded on the 30th plate with a loading capacity of 20 liters. The upper catalyst is loaded on the 60th tray with a loading capacity of 14 liters. The remainder of the tower is filled with 1.5 cm long porcelain rings. The upper bed catalyst selects the catalyst to include the following components in weight percent: (a) 20.0% NiO oxide; (b) 1.0% cerium oxide; (c) 0.2% magnesium oxide; (d) the remainder Alumina carrier. The catalyst in the lower bed layer is selected from a) 40% nickel or its oxide; b) 5% titanium oxide; c) the balance of carrier alumina; wherein the precursor of carrier alumina is aluminum sol. C4 material enters the reactive distillation column from the 29th tray, and hydrogen ent...
Embodiment 2
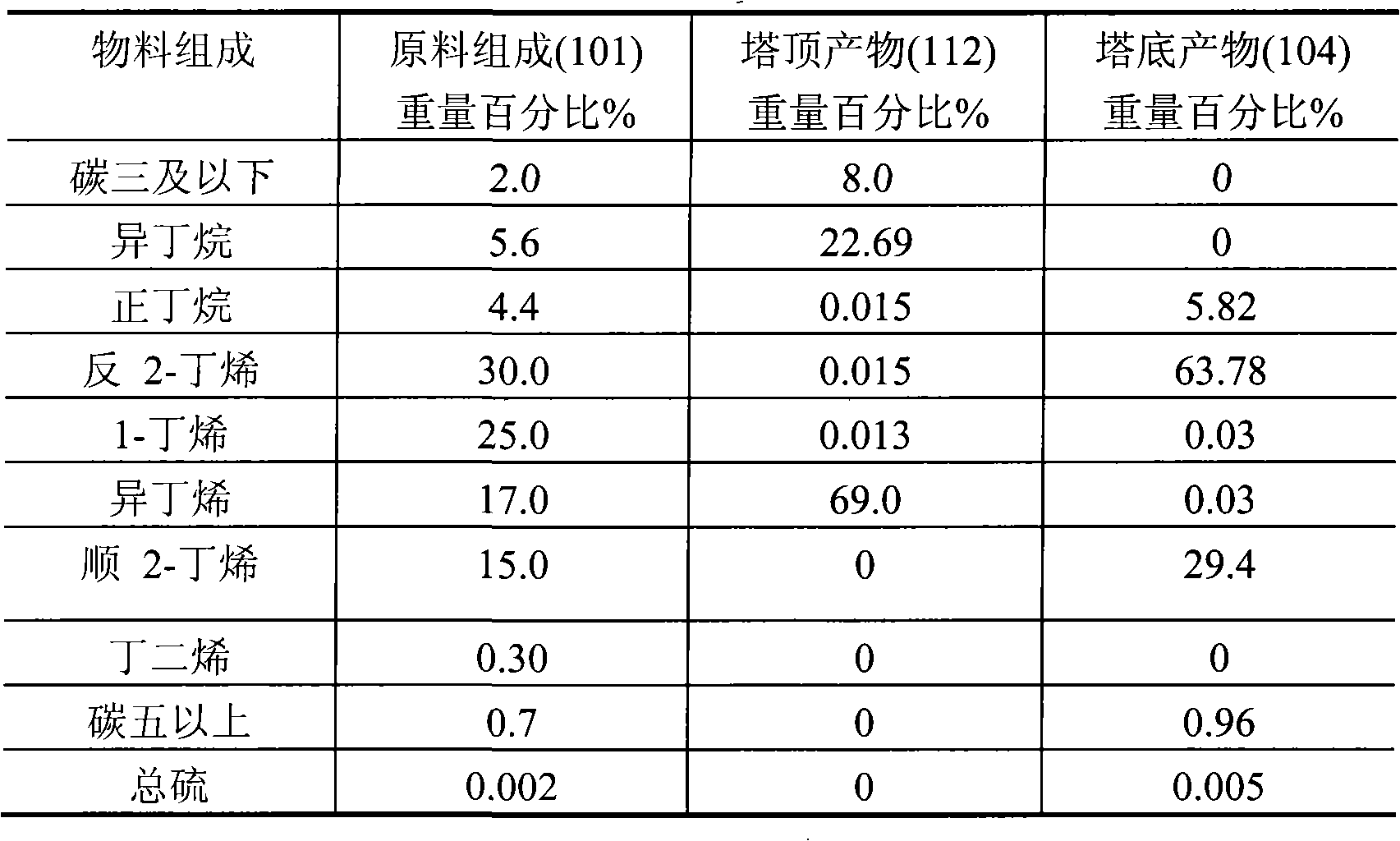
[0020] A reactive distillation column with a diameter of 10 cm and a height of 20 meters has a total of 100 trays from the bottom to the top of the column, numbered 1, 2, 3, ... 100 in sequence. One deck of catalyst is packed in the tower, and the catalyst is loaded on the 40th plate, and the loading capacity is 30 liters. The remainder of the tower is filled with 1.5 cm long porcelain rings. The catalyst is selected to include the following components in weight percent: (a) 30.0% NiO oxide; (b) 2.0% cerium oxide; (c) 0.2% magnesium oxide; (d) 3% titanium oxide ; (e) the remainder of the carrier alumina. The precursor of the carrier alumina is aluminum sol. C4 material enters the reactive distillation column from the 30th tray, and hydrogen enters from the 35th tray. The operating temperature at the bottom of the tower is 60°C, and the pressure at the bottom of the tower is 0.6MPa; the operating temperature at the top of the tower is 50°C, and the pressure at the top of the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com