Biodegradable polyurethane material based on piperazine block D, L-polylactic acid and preparation method thereof
A polyurethane material, polylactic acid technology, applied in the field based on piperazine block D, can solve the problems of difficult control of polyurethane synthesis process, lack of mechanical strength, aseptic inflammation, etc. Mechanical properties, the effect of improving mechanical properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Weigh 5.000 g (0.03472 mol, molecular weight: 144) of D,L-lactide that has been distilled and recrystallized from ethyl acetate three times according to the molar ratio of the substance at about 10:1, and the anhydrous piperine after vacuum drying. Zine 0.2988g (0.00347mol, molecular weight: 86.1) was added to a 25ml round-bottomed flask, 1 / 5000 of the molar amount of D, L-lactide substance was added with stannous octoate as an initiator, and then vacuumed for 2 ~3 times, pumped for 15min respectively, sealed the flask, placed in an oil bath to heat, controlled the temperature to 150°C, and reacted for 24h. The reaction product was dissolved in dichloromethane, purified with n-hexane, and dried at room temperature to obtain 3.823 g of polylactic acid with a hydroxy-terminated piperazine block.
Embodiment 2
[0031] Weigh 5.000 g (0.03472 mol, molecular weight: 144) of D, L-lactide that has been distilled and recrystallized from ethyl acetate three times according to the molar ratio of the substance at about 20:1, and anhydrous piperine after vacuum drying. Zine 0.1495g (0.00173mol, molecular weight: 86.1) was added to a 25ml round-bottomed flask respectively, and stannous octoate was added as an initiator by 1 / 5000 of the molar amount of D, L-lactide substance, and then vacuumed for 2 ~3 times, pumped for 15min respectively, sealed the flask, placed in an oil bath to heat, controlled the temperature to 150°C, and reacted for 24h. The reaction product was dissolved in dichloromethane, purified with n-hexane, and dried at room temperature to obtain 3.901 g of polylactic acid with a hydroxy-terminated piperazine block.
Embodiment 3
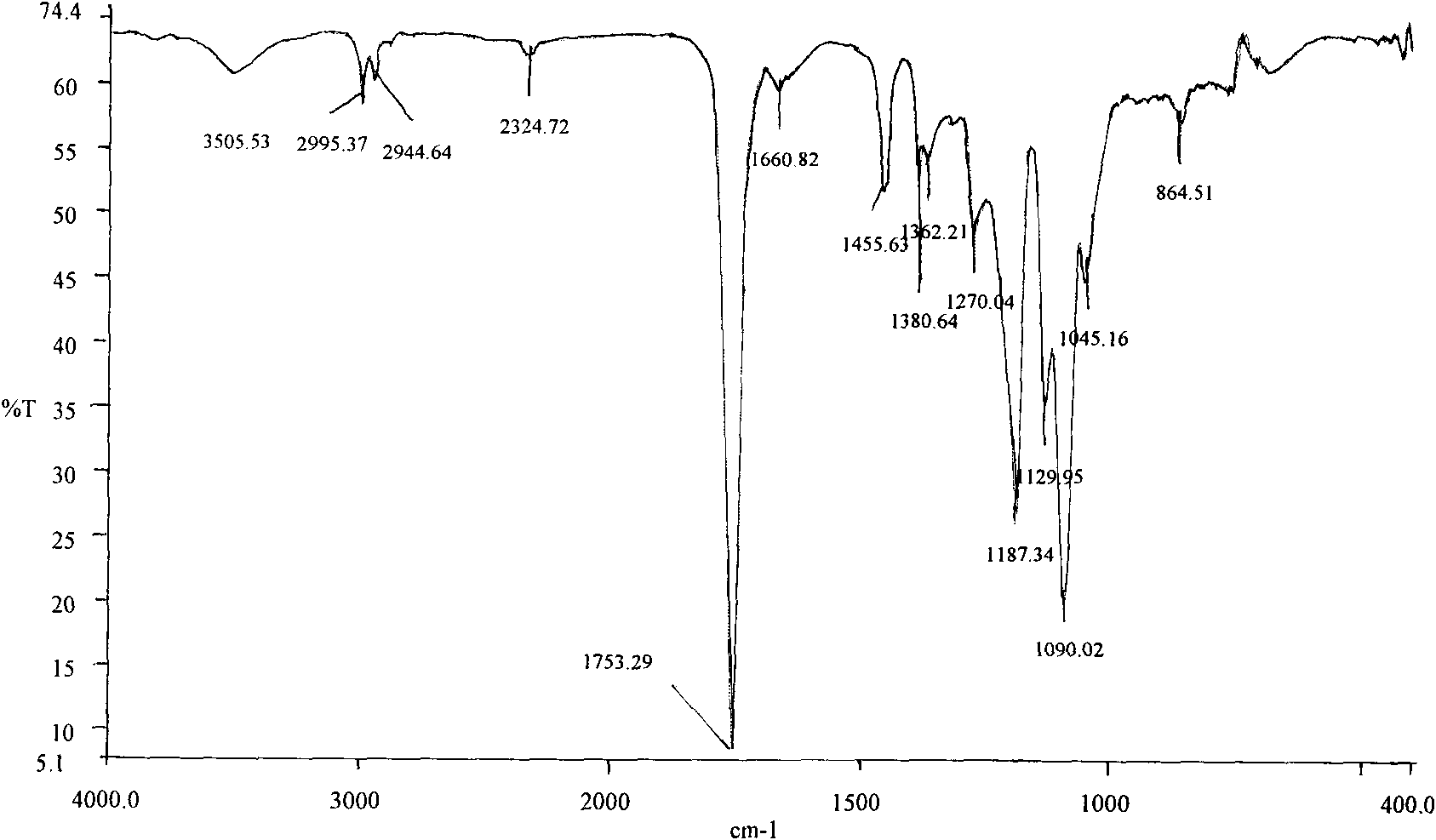
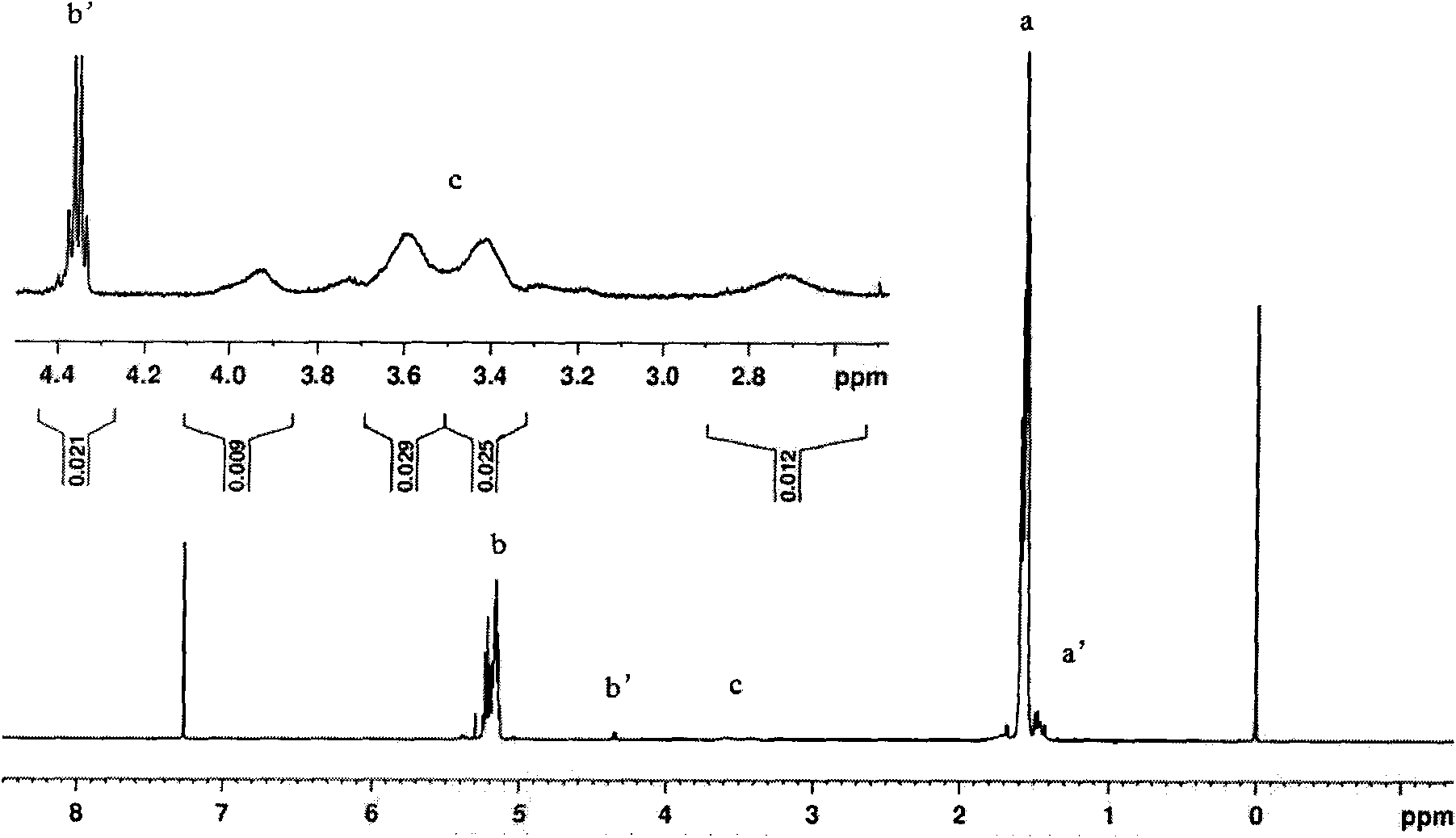
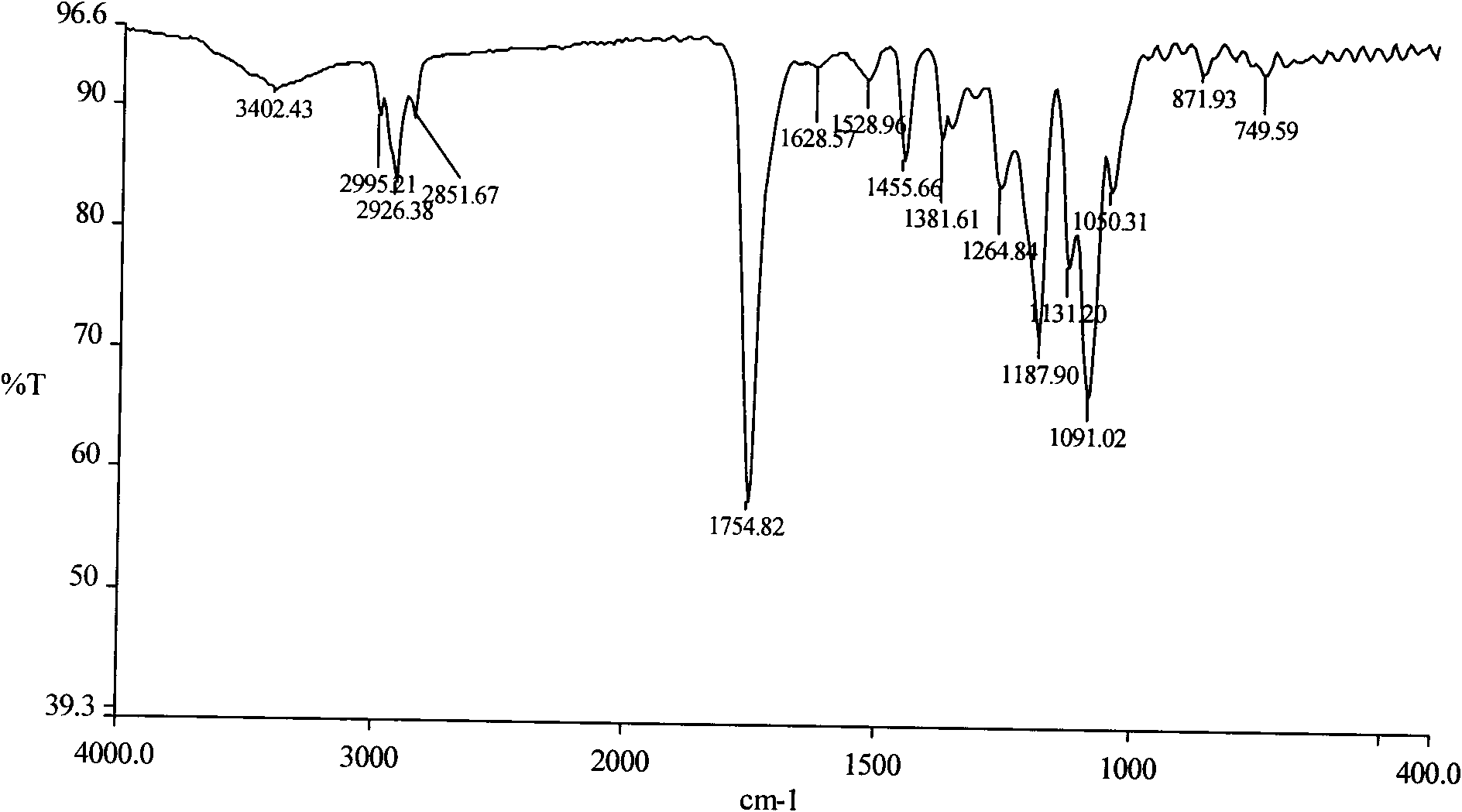
[0033] Weigh 5.000 g (0.03472 mol, molecular weight: 144) of D,L-lactide that has been distilled and recrystallized from ethyl acetate three times according to the molar ratio of the substance at about 30:1, and anhydrous piperine after vacuum drying. Zine 0.0996g (0.00116mol, molecular weight: 86.1) was added to a 25ml round-bottomed flask, 1 / 5000 of the molar amount of D, L-lactide substance was added with stannous octoate as an initiator, and then vacuumed for 2 ~3 times, pumped for 15min respectively, sealed the flask, placed in an oil bath to heat, controlled the temperature to 150°C, and reacted for 24h. After the reaction product was dissolved in dichloromethane, it was purified with n-hexane and dried at room temperature to obtain 4.147 g of polylactic acid with a hydroxy-terminated piperazine block. Its infrared spectrum see figure 1 , whose NMR images are shown in figure 2 .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Modulus | aaaaa | aaaaa |
| Modulus | aaaaa | aaaaa |
| Modulus | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com