Human umbilical cordmesenchymal stem cell-derived exosome and application thereof
A technology of mesenchymal stem cells and membranous vesicles, applied in drug research and development, can solve the problems of low survival rate of MSC transplantation, limitations, hidden dangers of tumorigenic clinical application, etc., and achieve the effect of simple preparation technical scheme, convenient storage, and easy control of use time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
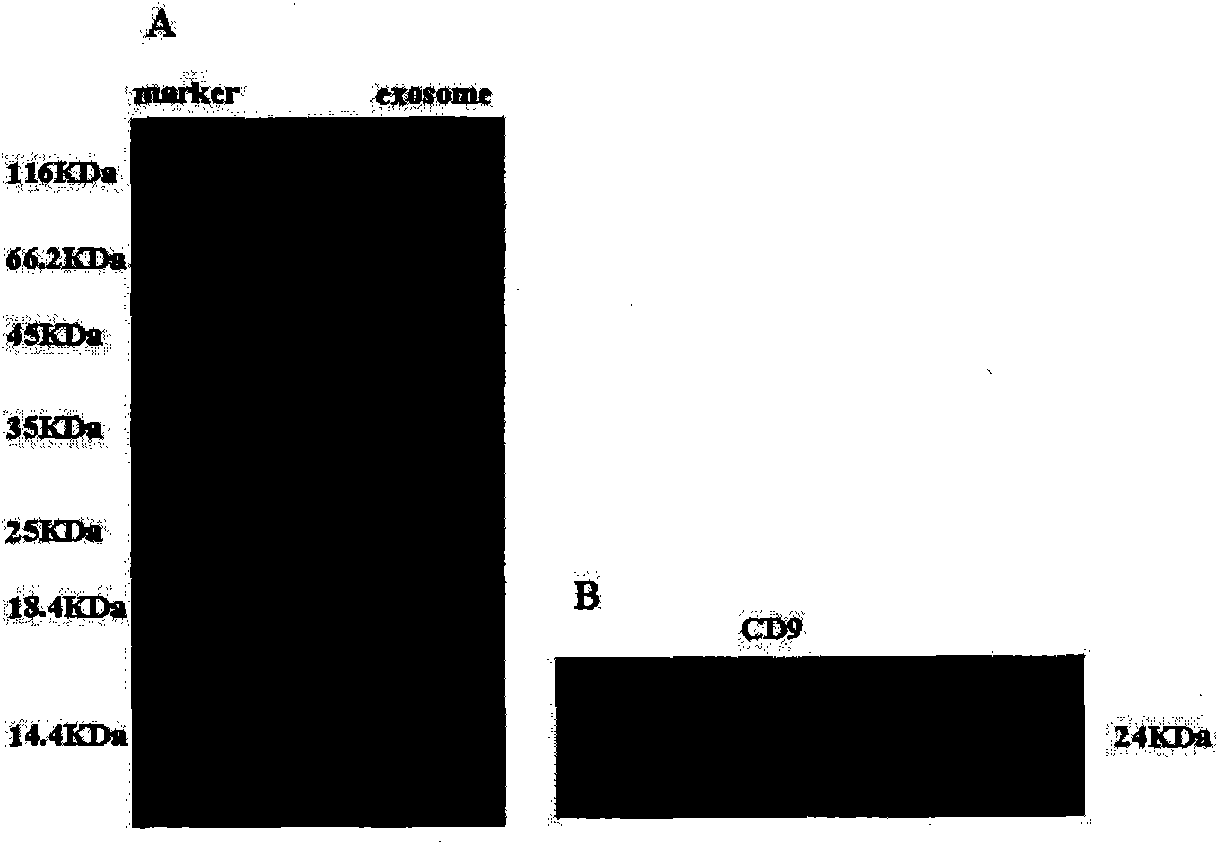
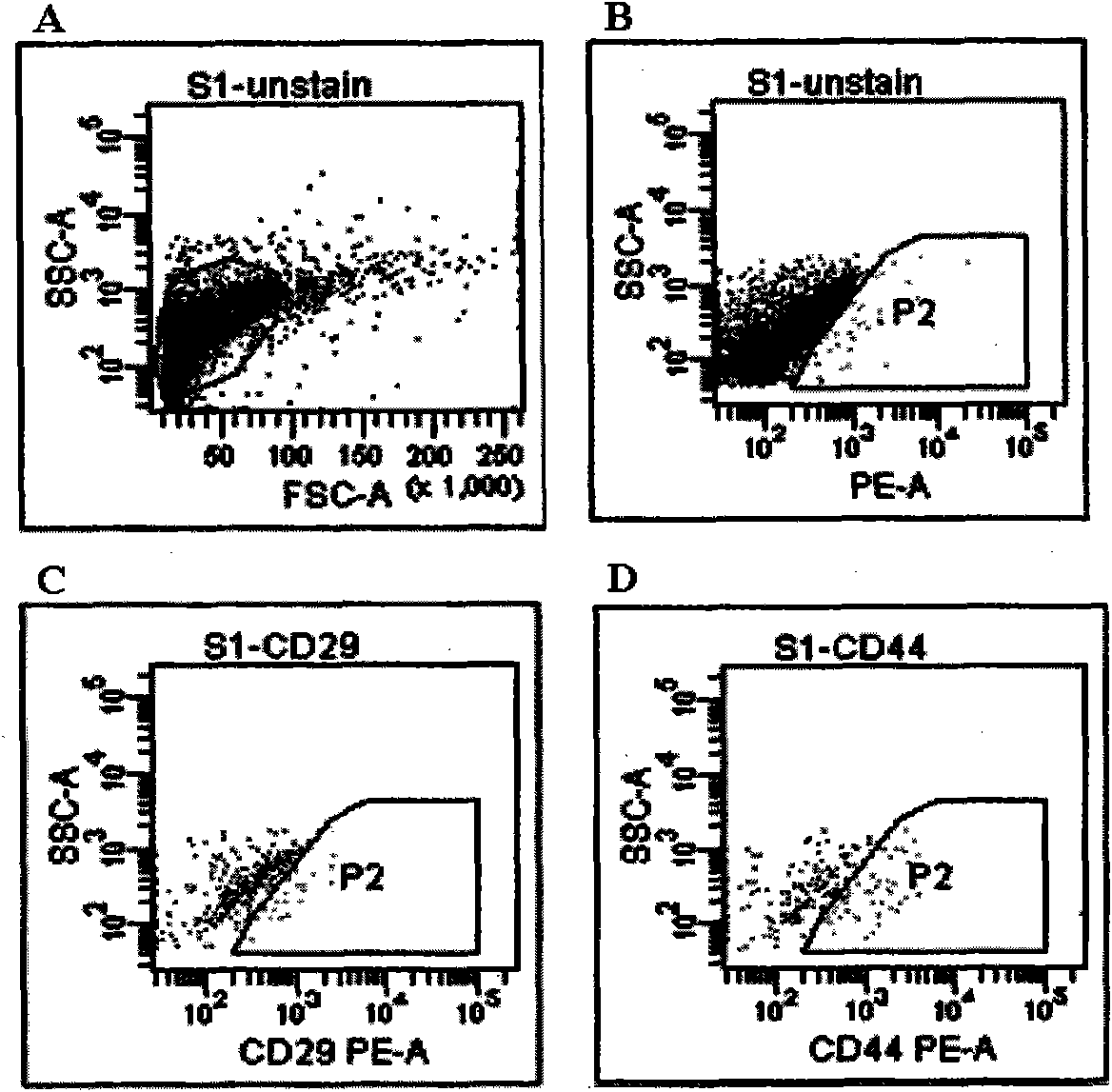
[0026] Example 1: Preparation of membranous vesicles derived from umbilical cord mesenchymal stem cells (hucMSC-exosome)
[0027] 1. Isolation and culture of human umbilical cord mesenchymal stem cells
[0028] Human umbilical cord MSCs were cultured and expanded in vitro according to the method previously established by our research group for isolation, culture and identification of human umbilical cord MSCs (Chun Qiao, et al. Cell Biology International, 2008, 32: 8-15). Specifically: take the fresh umbilical cord of a sterile newborn, wash it repeatedly with phosphate buffer saline (PBS), and cut it into tissue pieces with a diameter of about 1-2 mm; L-DMEM nutrient solution containing 10% fetal bovine serum, 5% CO , Culture in saturated humidity at 37°C; remove non-adherent cells, and digest 0.25% trypsin after 80% confluence of adherent cells for subculture.
[0029] 2. Preparation of Human Umbilical Cord Mesenchymal Stem Cell Conditioned Medium (MSC-CM)
[0030] Select ...
Embodiment 2
[0034] Example 2: Observation of curative effect of hucMSC-exosome on mouse 20% deep second-degree SD rat scald model
[0035] (1) Experimental animals were divided into groups and 20% deep second-degree scald model to prepare clean-grade SD rats, weighing about 120-130g, with a total of 6 rats, half male and half male, divided into control group, umbilical cord mesenchymal stem cell treatment group, and umbilical cord mesenchymal stem cell treatment group. Mesenchymal stem cell exosome treatment group, 2 rats in each group, one male and one male. The anesthetized rat was placed on the operating table, and the 20% body surface area of the rat was calculated according to the Rubner formula. Cut off the back hair, remove the hair with sodium thiosulfide, put it in 82°C water, take it out quickly after 8 seconds, dry it with gauze (to avoid further aggravating the wound damage), add 6ml of normal saline, and wrap it with sterile gauze .
[0036] (2) Treatment method 3 hours a...
Embodiment 3
[0038] Example 3: Repairing effect of hucMSC-exosome on rat renal tubular epithelial cells (NRK) hypoxia model
[0039] Establishment of NRK cell hypoxia model: NRK cells were anoxic for 4 hours under the condition of 5% CO2, 2% O2, 37°C and saturated humidity, and after adding exosome for 48 hours, various indicators were detected.
[0040] Dosage:
[0041] Low dose group (0.5mg / 5×10 5 cells)
[0042] Middle dose group (1mg / 5×10 5 cells)
[0043] High dose group (2mg / 5×10 5 cells)
[0044] Using flow cytometry, TUNEL, and immunohistochemistry to evaluate the repair effect of exosome secreted by hucMSCs on NRK cell hypoxia model in vitro. Quantitative PCR, Western blot and other techniques were used to preliminarily explore the main mechanism of damage repair. After hypoxia / reoxygenation or cisplatin treatment in NRK cells, the nuclei shrunk, TUNEL was deeply stained, and the cell shape was large and irregular. After adding exosome, the cell morphology was better, and ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com