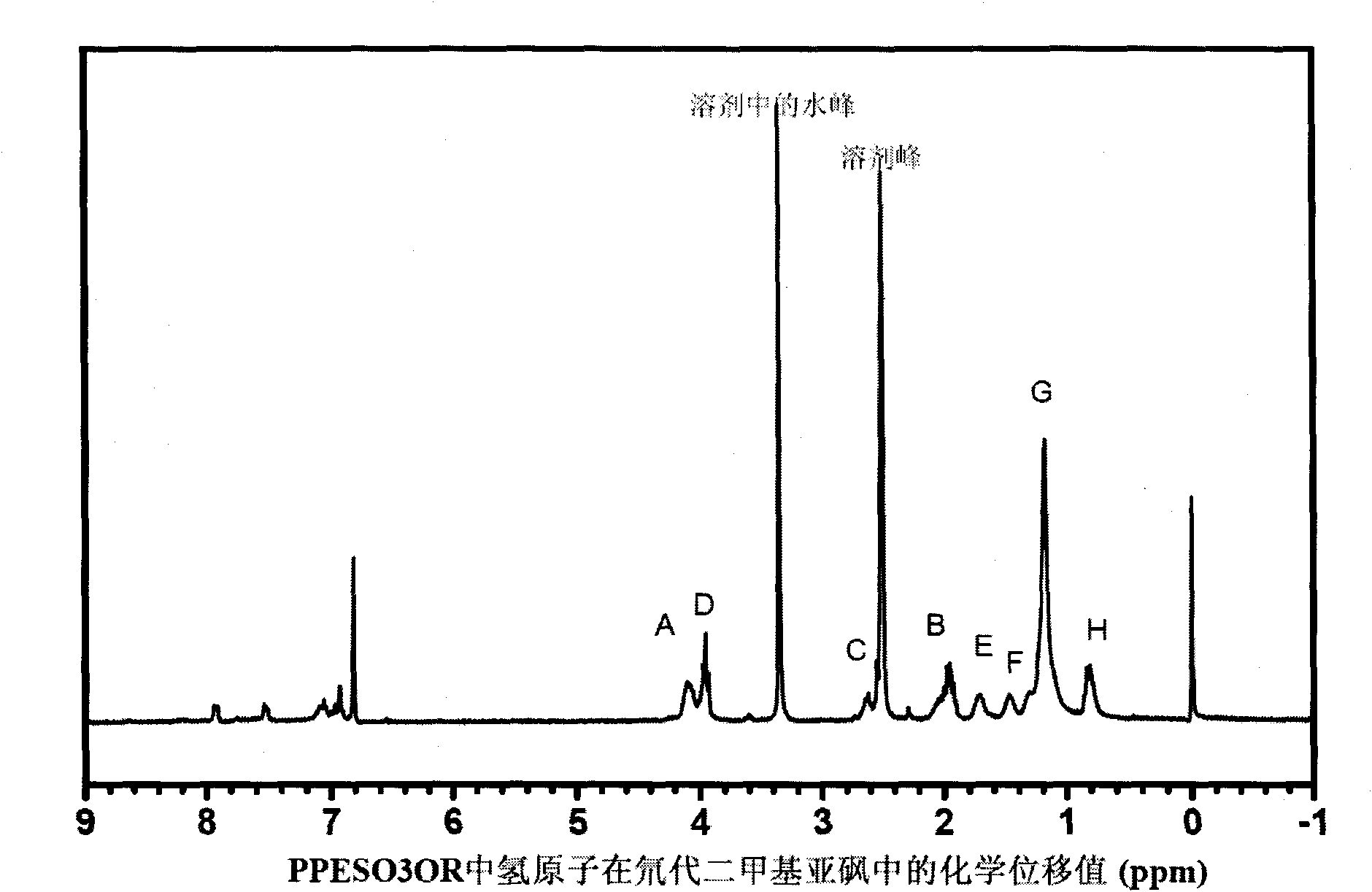
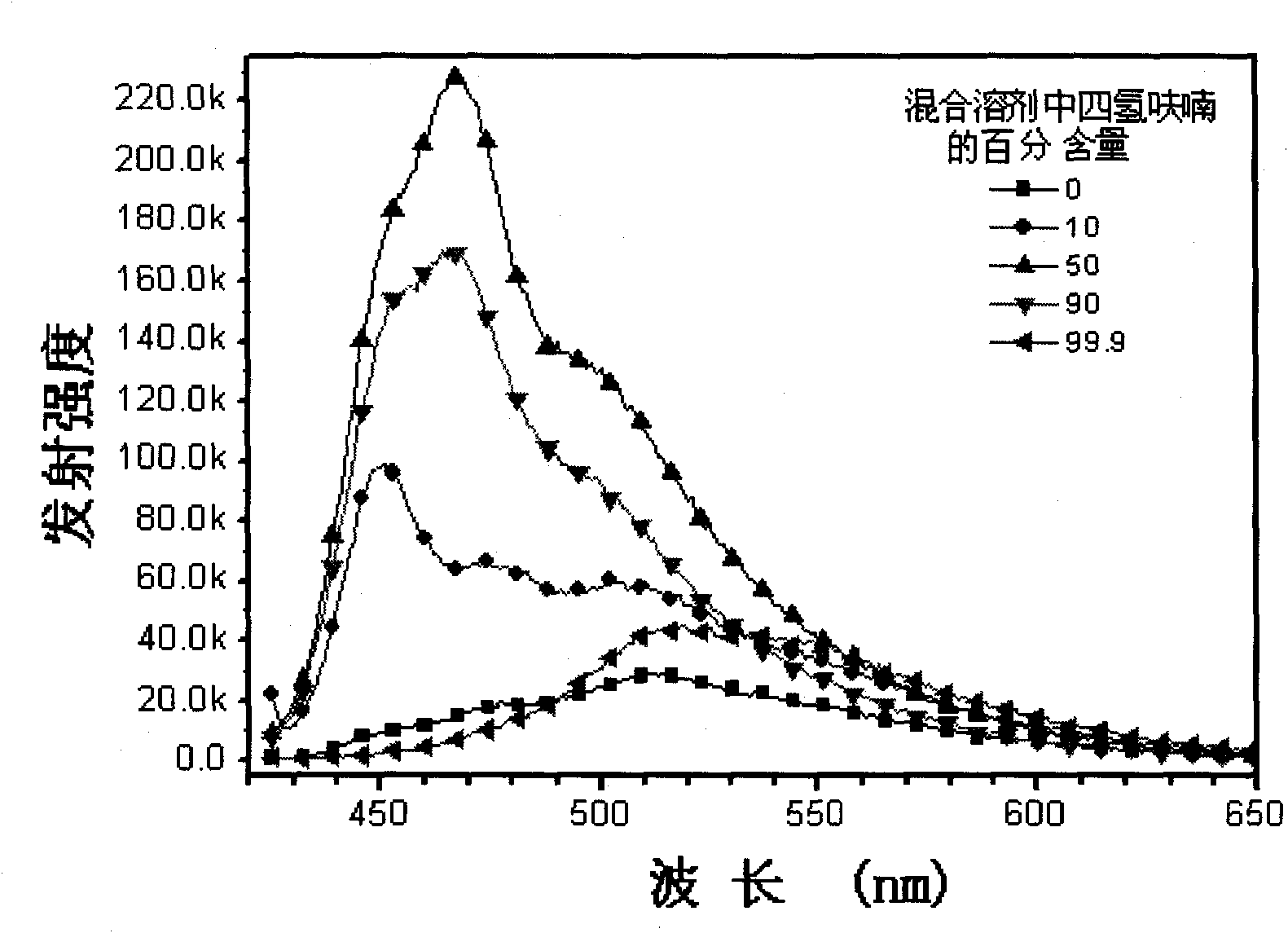
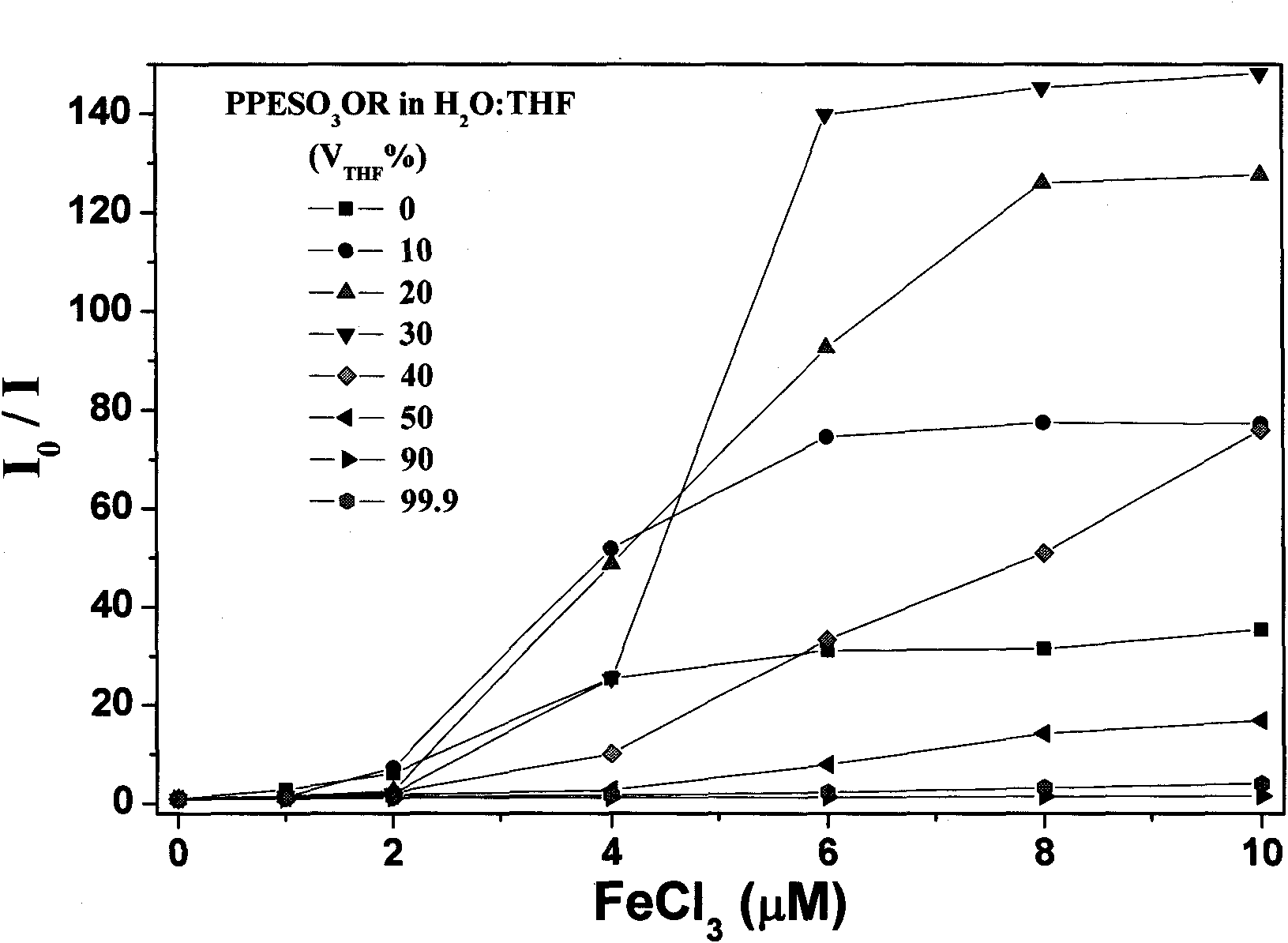
Fluorescence conjugated polyelectrolytes with amphoteric side chain, preparation method and application
A technology of conjugated polyelectrolytes and side chains, which is applied in the field of fluorescent chemical sensing materials, can solve problems such as difficulties in storage and transportation, affecting luminescent performance and sensing efficiency, and reducing the efficiency of conjugated high molecular weight molecules, so as to achieve convenient storage and transport effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The oil-soluble monomer used in this example is 1,4-diethynyl-2,5-docosyloxybenzene; the water-soluble monomer used is 1,4-diiodo-2,5-dipropane Benzene oxysulfonate.
[0030] (1) The synthesis steps of 1,4-diethynyl-2,5-docosyloxybenzene are as follows:
[0031] Preparation of 1,4-docosyloxybenzene
[0032] A DMF solution containing hydroquinone (11 g, 100 mmol) and dodecane bromide (2.2 equivalents) was placed in a 500 ml single-necked flask equipped with a condenser, and potassium hydroxide (250 mmol) was added thereto. The mixed solution was stirred and slowly heated to 120° C., and stirred and refluxed after the reaction was stable. After finishing the reaction, the reaction solution was cooled to room temperature, and the solid precipitate was collected by suction filtration. The collected solid was recrystallized with ethanol to obtain a white solid, which was dried in a vacuum oven to obtain the pure product 1,4-docosyloxy phenyl (85% yield).
[0033] Preparati...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com