Pegylation hypoglycemic polypeptide and preparation method and application thereof
A technology for PEGylation and hypoglycemic peptides, which is applied in the preparation methods of peptides, chemical instruments and methods, biochemical equipment and methods, etc. Improve medication compliance, high stability, and pain relief
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054] Recombinant expression and preparation of PEGylated hypoglycemic polypeptide
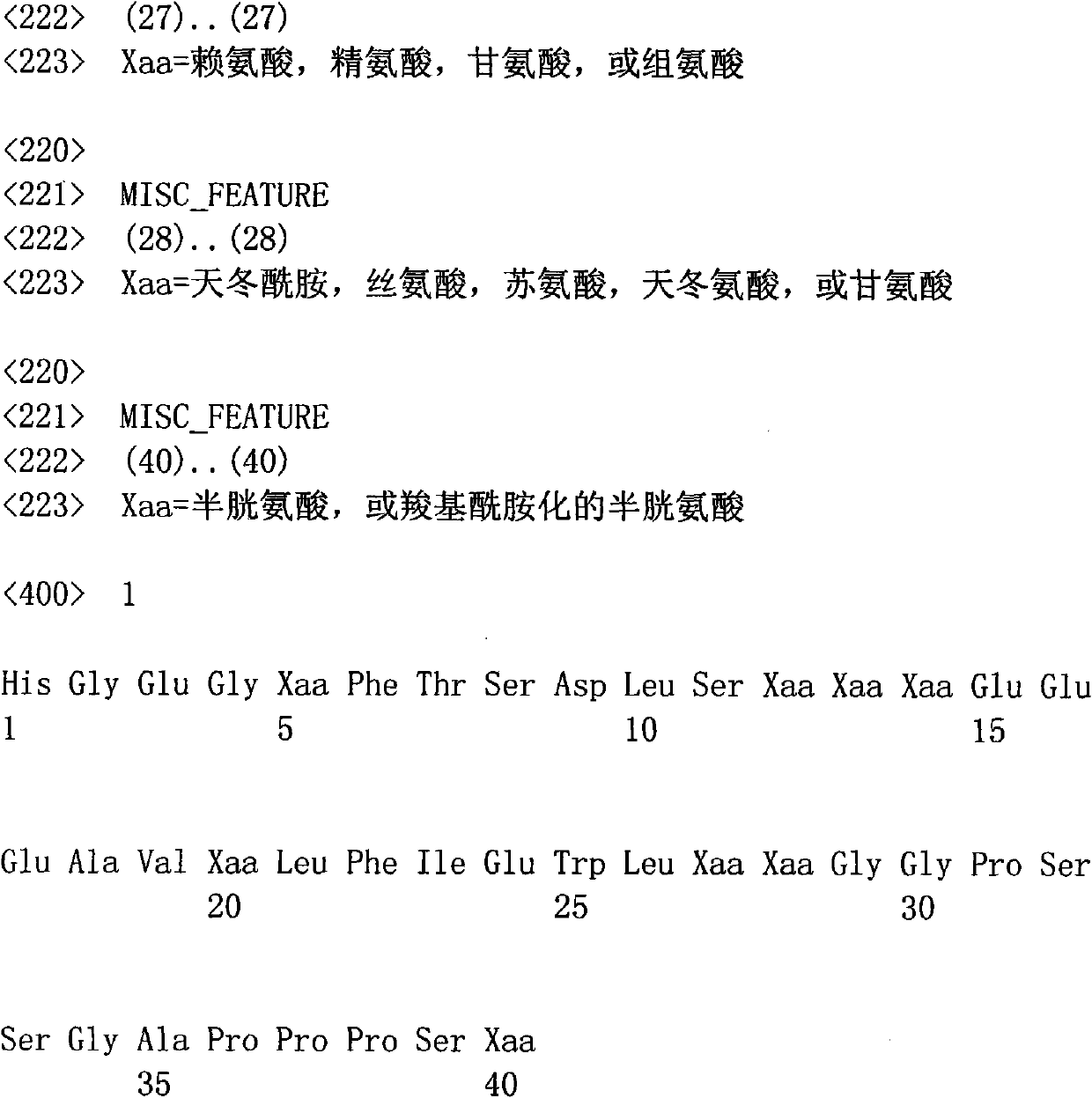
[0055] According to the amino acid sequence of the hypoglycemic polypeptide according to claim 1, its amino acid sequence is as follows:
[0056] His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp- Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser-Cys.
[0057] Corresponding DNA sequences were designed and synthesized according to the codon table of Escherichia coli and considering its codon preference. The final design and optimization of the resulting DNA sequence is as follows:
[0058] 5'-CACGGTGAAGGTACTTTTCACCTCTGACCTGTCTAAACAGATGGAAGAAGAAGCTGTTCGTCTGTTCATCGAATGGCTGAAAAACGGTGGTCCGTCTTCTGGTGCTCCACCGCCATCTTGC-3'.
[0059] Add the DNA sequence corresponding to the aspartic acid-aspartic acid-aspartic acid-aspartic acid-lysine sequence at the 5' end of the sequence, and add a TAA stop codon at its 3' end. This fragment was inserted i...
Embodiment 2
[0067] Solid Phase Synthesis of Pegylated Hypoglycemic Peptides
[0068] According to the amino acid sequence of the hypoglycemic polypeptide according to claim 1, its sequence is as follows:
[0069] His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp- Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Pro-Ser-Cys (CONH 2 ),
[0070] Among them, Cys(CONH 2 ) is carboxyl amidated cysteine. The Fmoc-protected amino acid is used for solid-phase synthesis reaction, and the whole reaction is completed on a peptide solid-phase synthesizer.
[0071] The synthesized polypeptide is purified by preparative reverse-phase liquid chromatography to obtain the target hypoglycemic polypeptide with a purity higher than 98%, and the physical molecular weight of the obtained polypeptide is detected by ESI-MS. The measurement results showed that the physical molecular weight of the obtained polypeptide was 4289.7Da. Tricine-SDS-PAGE test res...
Embodiment 3
[0097] Analysis of Physicochemical Properties of Pegylated Hypoglycemic Peptides
[0098] The amino acid sequence structure of the hypoglycemic polypeptide is as follows:
[0099] His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser-Lys-Gln-Met-Glu-Glu-Glu-Ala-Val-Arg-Leu-Phe-Ile-Glu-Trp- Leu-Lys-Asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Por-Ser-Cys (CONH 2 ),
[0100] Among them, Cys(CONH 2 ) is carboxyl amidated cysteine. 5kDa, 10kDa, 20kDa, 30kDa, and 40kDa maleimide-activated polyethylene glycol were used to modify the polypeptide with polyethylene glycol, and the obtained pegylated hypoglycemic polypeptides were named: PEG5K-GP, PEG10K-GP, PEG20K-GP, PEG30K-GP, and PEG40K-GP.
[0101] The detection methods for their physical and chemical properties are the same, and only PEG20kDa modified glucose-lowering peptide (PEG20K-GP) is used as an example to illustrate below.
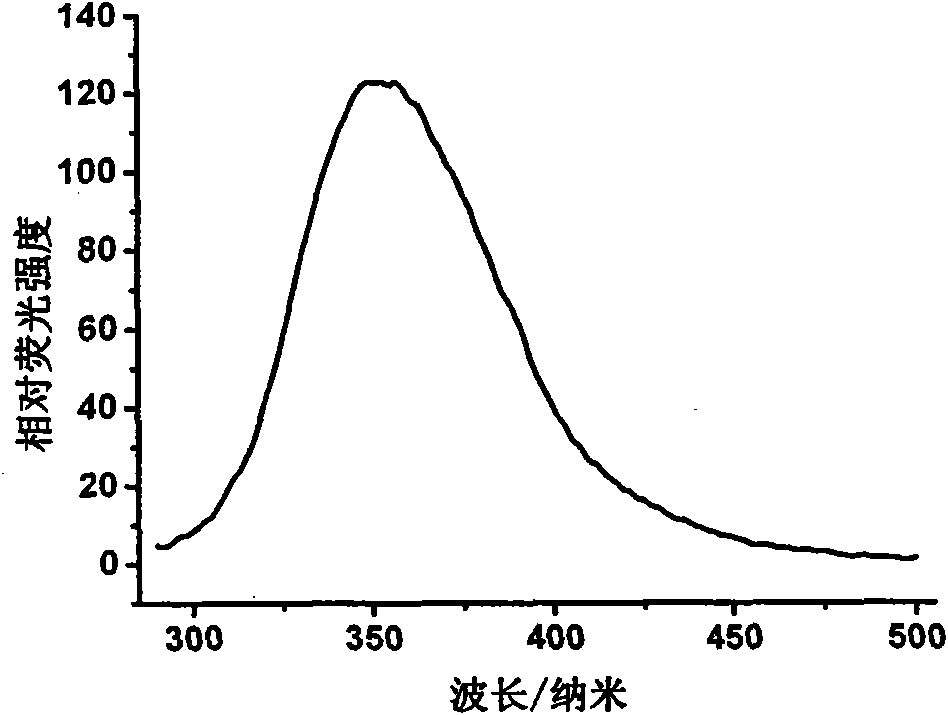
[0102] Purity analysis. The chromatographic purity and electrophoretic purity of the obtained PEGylated hypo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Apparent molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com