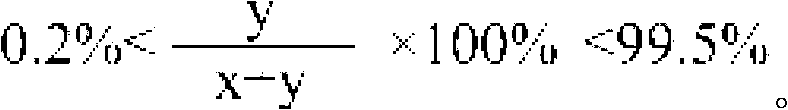Polyester and preparation method thereof
A technology of polyester and polymerization degree, which is applied in the direction of pharmaceutical formula, medical preparation of non-active ingredients, medical science, etc., to achieve the effect of high efficiency and good hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
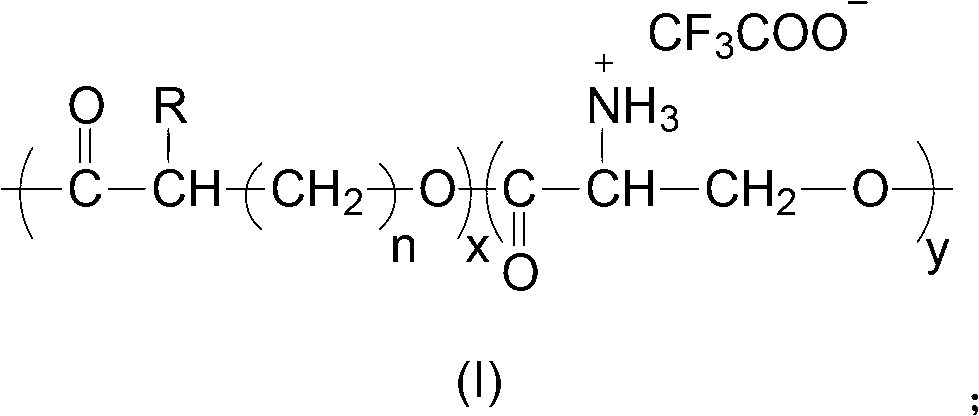
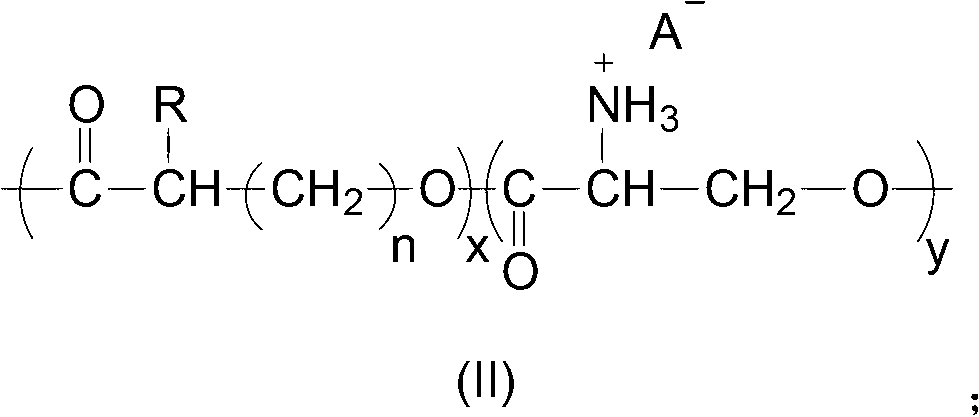
[0041] The present invention also provides a method for preparing the polyester having the structure of formula (I), including:
[0042] Using diethyl zinc as a catalyst, the aliphatic polyester and N-tritylserine lactone are polymerized to form a copolymer, and the aliphatic polyester is polyglycolide, polycaprolactone or polylactide;
[0043] The copolymer is mixed with trifluoroacetic acid, and reacts at 0°C to 35°C to produce a polyester having a structure of formula (I).
[0044] In the present invention, aliphatic polyester and N-tritylserine lactone are used as raw materials to prepare a polyester having a structure of formula (I). The aliphatic polyester is polyglycolide, polycaprolactone or polylactide, which can be purchased from the market. The N-tritylserine lactone is preferably prepared according to the following method:
[0045] Mixing serine and trityl chloride, reacting in an organic solvent to obtain N-tritylserine, the organic solvent being a mixed solution of dich...
Embodiment 1
[0085] Example 1 Synthesis of N-tritylserine lactone
[0086] Under the protection of nitrogen, mix 6.3g serine, 100mL dichloromethane and 26mL trimethylchlorosilane, reflux for 1h at 50℃, cool to room temperature, add a mixture consisting of 29.4mL triethylamine and 60mL dichloromethane, and warm up Reflux at 50°C for 45 minutes, cool to 0°C, then add a mixture consisting of 3.6mL methanol and 15mL dichloromethane, 8.4mL triethylamine and 16.7g trityl chloride. After stirring for 12h at room temperature, add 12mL methanol And 8.4 mL of triethylamine, stirred for 15 min to obtain a mixed solution; remove the solvent and volatile substances in the mixed solution under vacuum to obtain a light yellow substance; use 300 mL of ethyl acetate to dissolve the light yellow substance, and use 150 mL of a 5% citric acid aqueous solution with a mass concentration of 5% was washed three times, and then three times with water. The obtained solution was rotary evaporated to obtain a crude prod...
Embodiment 2
[0088] Example 2 Synthesis of poly(glycolide-serine ester)
[0089] 0.522g (4.5mmol) of glycolide, 0.1645g (0.5mmol) of N-tritylserine lactone prepared in Example 1, 1.5mL of toluene and Diethylzinc with a molar ratio of 1:100 to N-tritylserine lactone, reacted at 90°C for 12 hours, quickly cooled the reaction flask, and removed the solvent. The product was dissolved in chloroform and then settled in methanol. , And then filtered, washed, and dried to obtain poly(glycolide-N-polytritylserine ester);
[0090] Add 0.2087g of poly(glycolide-N-polytritylserine ester), 10mL of dichloromethane, 0.0125mL of trifluoroacetic acid and 0.03mL of methanol to the flame-baked reaction flask, stir to dissolve the mixture, After reacting at 0°C for 3 hours, the product was concentrated and then settled in ether to obtain poly(glycolide-serine ester), in which the molar percentage of the serine ester repeating unit was 10%.
[0091] The poly(glycolide-serine ester) was measured by gel permeation ch...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
| molecular weight distribution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com