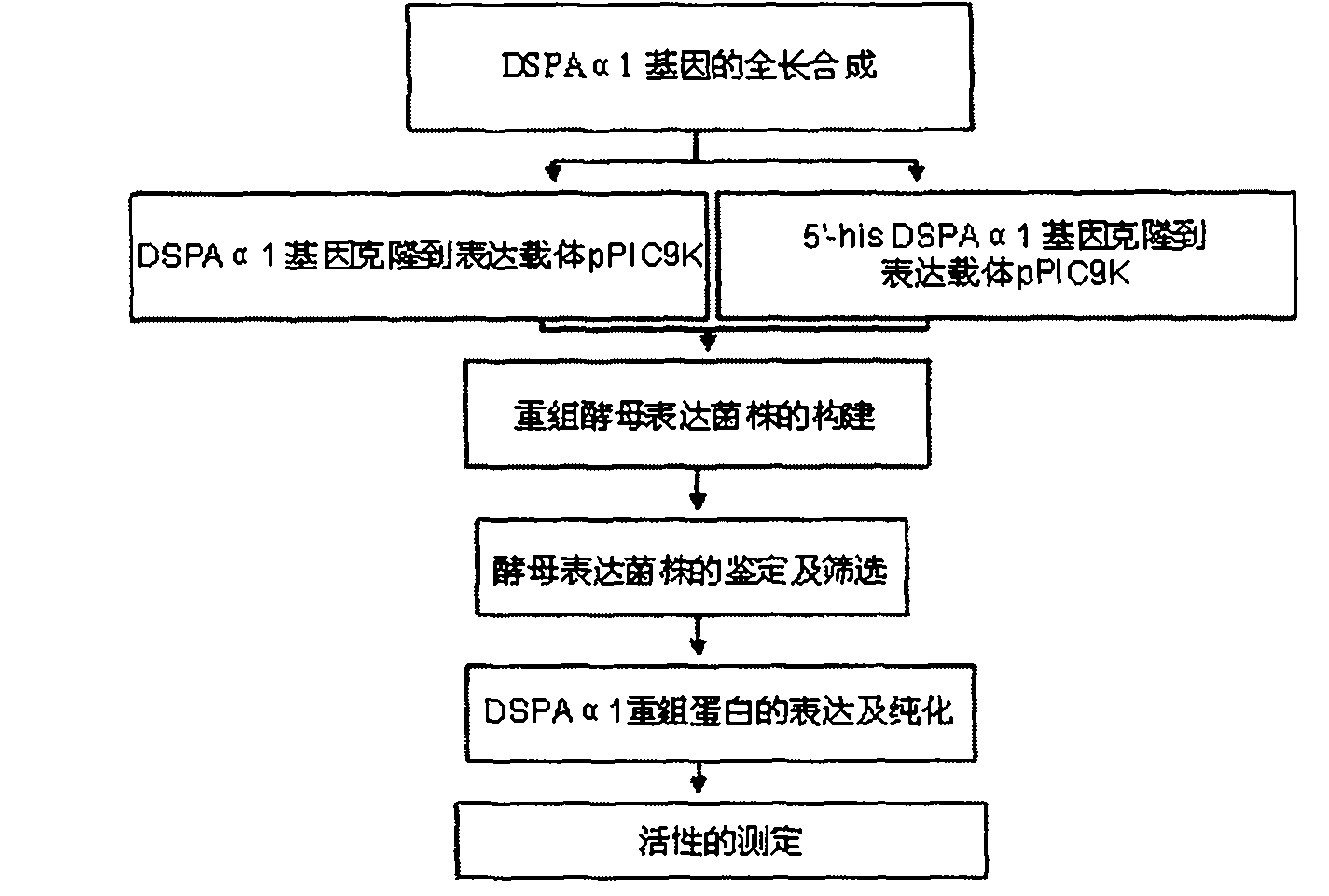
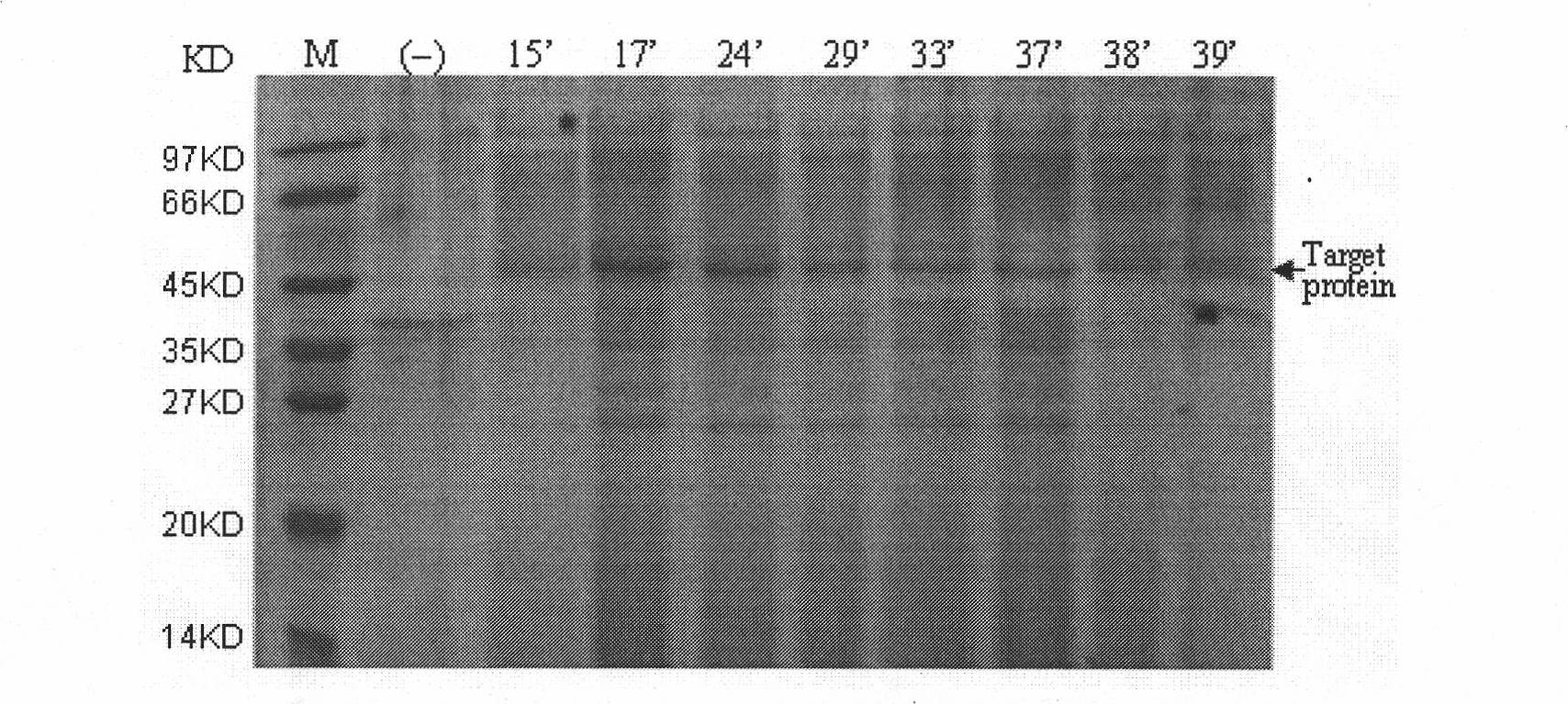
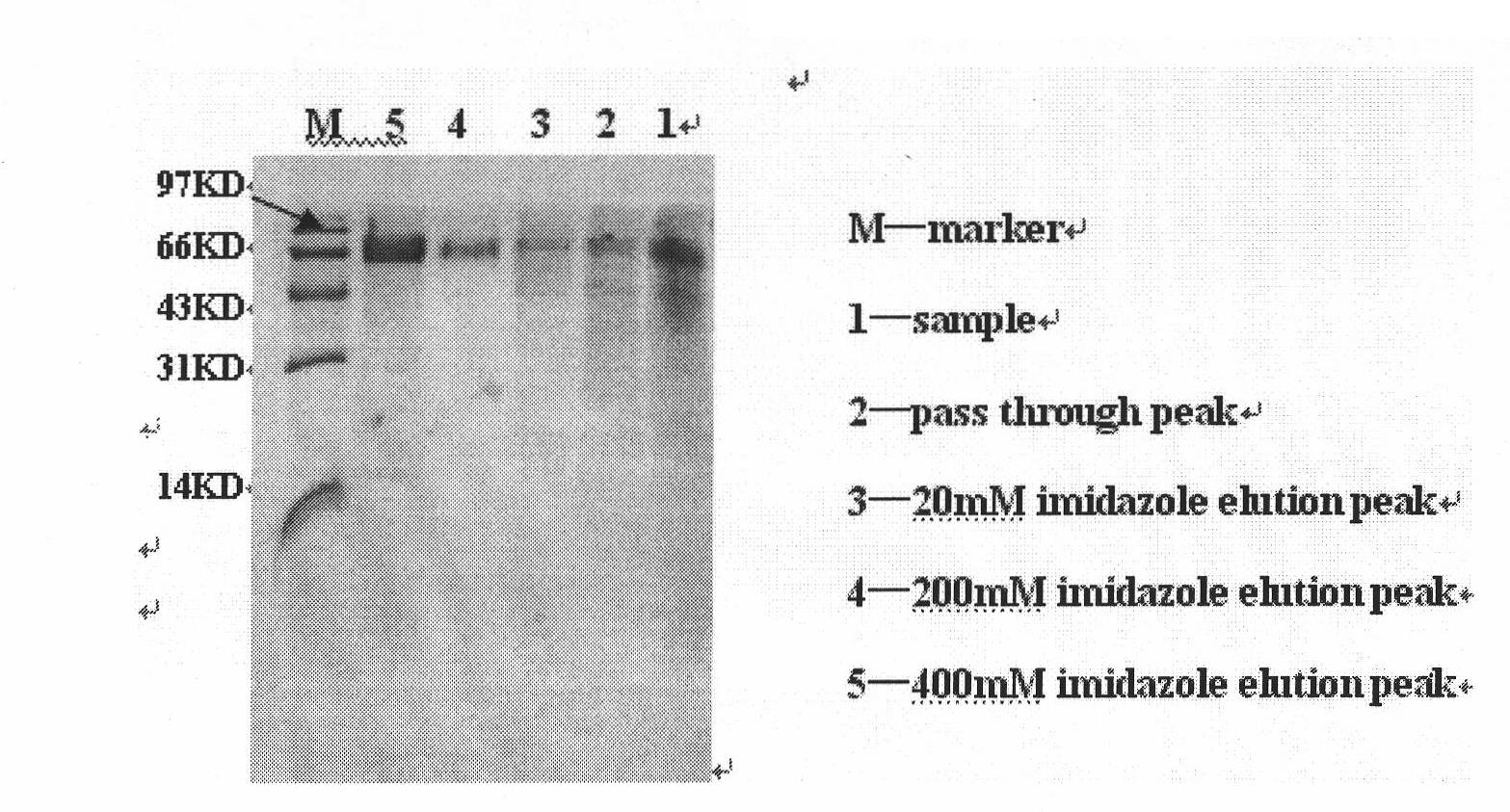
Method for expressing desmodus rotundus salivary plasminogen activator in pichia pastoris
A technology of plasminogen and Pichia pastoris, which is applied in the field of genetic engineering, can solve the problems such as unfavorable eukaryotic protein expression, low expression amount and high expression cost of Escherichia coli expression system
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1: Artificial synthesis of DSPAα1 gene
[0033] DSPAα1 was obtained from Gene Bank, its own signal peptide sequence was removed, and for the convenience of cloning into the pPIC9K plasmid in the next step, an Xho I restriction site was designed at the 5′ end, and an EcoR I restriction restriction site was designed at the 3′ end The site, a total of about 1340bp, was artificially synthesized in full length by Shanghai Sangong in vitro, cloned into the pUC57 vector, and transformed into E. coli TOP10 for stable storage.
[0034] Gene fragments:
[0035] 5'CG CTCGAG AAAAGA coded GC
[0036] Xho I
[0037] ATATGGTGTG GCCTGCAGAG ACGAAAAAAC CCAGATGATA TACCAGCAAC AAGAGTCGTG GCTGCGCCCC
[0038] GAGGTCAGAA GCAAGCGGGT AGAACACTGC CGGTGCGATA GAGGATTGGC CCAGTGTCAC ACCGTGCCTG
[0039] TCAAAAGTTG CAGTGAACTG AGGTGCTTCA ATGGGGGGAC ATGCTGGCAG GCTGCATCTT TCTCAGACTT
[0040] TGTCTGTCAG TGCCCTAAAG GATATACGGG GAAACAGTGT GAAGTAGATA CCCATGCCAC CTGCTACAAG
[0041] GACCAGGGTG TCA...
Embodiment 2
[0058] Embodiment 2: the construction of carrier
[0059] (1) Amplification of the target gene DSPAα1
[0060] Use upstream primer: 5′-CCG CTCAGA AAAAGAGCATATGGTGTGGCCTGC-3'
[0061] Xho I
[0062] Downstream primer: 5′-GCGCGCG GAATTC TTATGGGCGCATGTTGTCTCG-3′
[0063] EcoR I
[0064] Using the plasmid pUC57 / DSPAα1 as a template, the target gene DSPAα1 was amplified by PCR
[0065] (2) Amplification of 5'His DSPAα1 gene
[0066] Up primer (5' end primer):
[0067] CCG CTCGAG AAAAGA GCATATGGTGTGGCCTGC
[0068] XhoI His Tag EK restriction site
[0069] Down primer (3' end primer): GCGCGCG GAATTC TTATGGGCGCATGTTGTCTCG
[0070] EcoR I
[0071] The 5'His DSPAα1 gene was amplified by PCR using the plasmid pUC57 / DSPAα1 as a template.
[0072] (3) Cloning the target gene DSPAα1, 5'His-DSPAα1 into the expression vector pPIC9K
[0073] 1) The target genes DSPAα1 and 5'His-DSPAα1 amplified by PCR were simultaneously digested with Xho I and EcoR I respectively, complet...
Embodiment 3
[0076] Example 3: Construction of DSPAα1, 5'His-DSPAα1 Pichia pastoris expression strain
[0077] (1) Linearization of DSPAα1 / pPIC9K, 5'His-DSPAα1 / pPIC9K
[0078] First, a large amount of DSPAα1 / pPIC9K and 5'His-DSPAα1 / pPIC9K were extracted from Escherichia coli TOP10 by alkaline lysis method, and then the above-mentioned plasmid DNA was digested with excess restriction endonuclease Sal I to make it completely linearized, and the agar After sugar gel electrophoresis, the digested products were recovered using a gel recovery kit (TIANGEN) and stored in a -20°C refrigerator for later use.
[0079] (2) Transformation of yeast cells
[0080] Take 100 μL of Pichia pastoris GS115 (purchased from Invitrogen, USA) stored in a refrigerator at -20°C, inoculate in 3mL LYPD medium and shake overnight at 30°C, take 2.5mL of the activated bacterial liquid and insert it into 250mL LYPD liquid medium and shake overnight at 30°C , to OD600=1.3-1.5, collect the cells to prepare competent cells;...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com