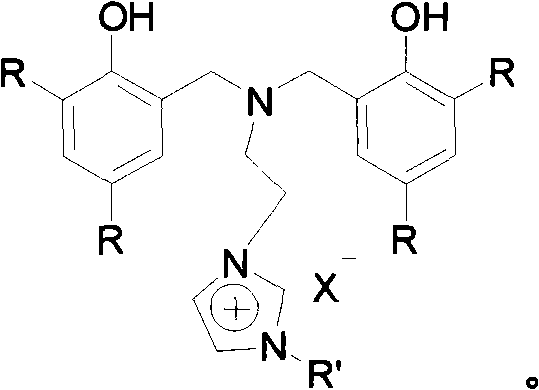
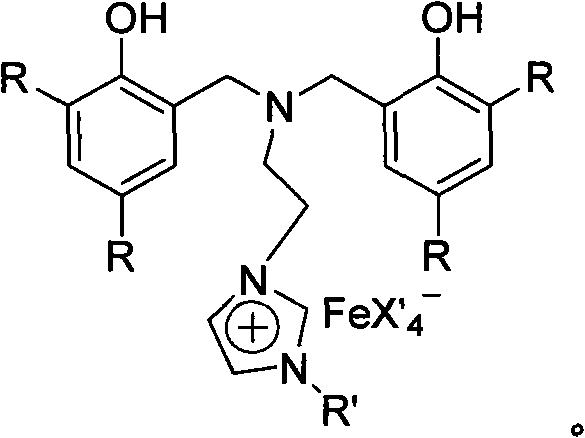
Nitrogen bridged bisphenol functionalized imidazole and ionic iron (III) complex thereof
A technology of imidazolium salt and imidazolium salt chemistry, applied in the direction of hydrocarbons, hydrocarbons, iron organic compounds, etc., can solve problems such as inconvenience of operation, and achieve the effects of eliminating interference, high yield and easy product
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Example 1: N, N-di(3,5-di-tert-butyl-2 hydroxybenzyl)-2-hydroxyethylamine {[3,5-di-C(CH 3 ) 3 -2-OH-C 6 h 2 ]CH 2} 2 NCH 2 CH 2 Synthesis of OH
[0043] Add 2,4-di-tert-butylphenol (20g, 98.4mmol), formaldehyde (7.4mL, 98.4mmol) and 2-hydroxyethylamine (3mL, 49.2mmol) to methanol in turn, heat and reflux for six days, and the product is It was precipitated from the system, filtered with suction, washed with methanol, and the yield was 62%.
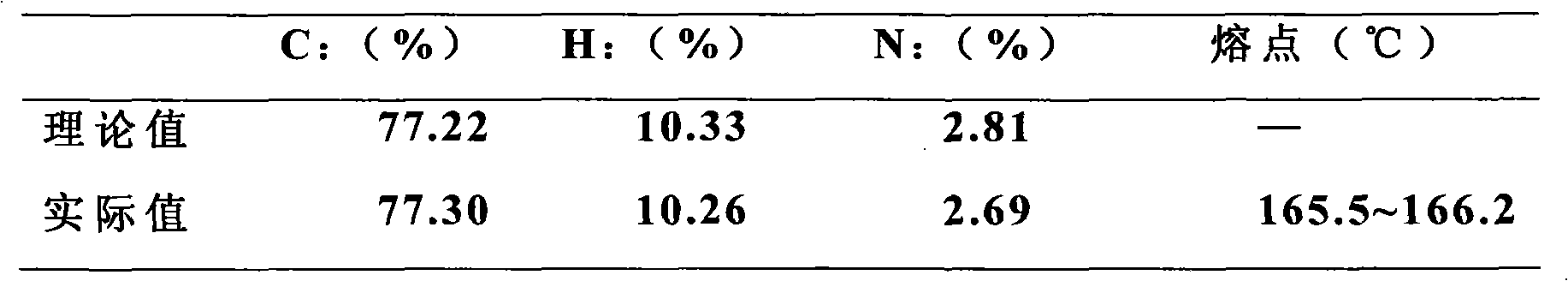
[0044] Carry out elemental analysis to product, test melting point, NMR analysis, the result is as follows:
[0045] Elemental analysis and melting point
[0046]
[0047] NMR data, 1 HNMR (CDCl 3 , δ, ppm) 8.40 (s, 2H, O-H), 7.18-7.21 (d, 2H, Ph-H), 6.86-6.91 (d, 2H, Ph-H), 3.84-3.90 (t, 2H, OCH 2 C), 3.74(s, 4H, PhCH 2 N), 2.70-2.75(t, 2H, NCH 2 C), 1.38(s, 18H, C(CH 3 ) 3 ), 1.25(s, 18H, C(CH 3 ) 3 ).
[0048] It was proved that the obtained compound was the target compound.
Embodiment 2
[0049] Example 2: N, N-di(3,5-di-tert-butyl-2 hydroxybenzyl)-2-chloroethylamine {[3,5-di-C(CH 3 ) 3 -2-OH-C 6 h 2 ]CH 2} 2 NCH 2 CH 2 Synthesis of Cl
[0050] (1) Dissolve N,N-bis(3,5-di-tert-butyl-2-hydroxybenzyl)-2-hydroxyethylamine (7.5g, 15mmol) in 100mL of anhydrous ether, Slowly add thionyl chloride (3.26mL, 41.25mmol) dropwise, after 30 minutes, slowly raise the temperature to 45°C, reflux for 3 hours, N,N-bis(3,5-di-tert-butyl-2-hydroxybenzyl Base)-2-chloroethylamine hydrochloride was precipitated from the system, filtered by suction, washed with ether, and the yield was 91%.
[0051] (2) Diethyl ether was added to N,N-bis(3,5-di-tert-butyl-2-hydroxybenzyl)-2-chloroethylamine hydrochloride, and then aqueous sodium bicarbonate was slowly added dropwise thereto. When the white solid in the solution disappears, stop the dropwise addition, wash three times with distilled water, and use 50 mL for one time, collect the ether layer, add an appropriate amount of anhyd...
Embodiment 3
[0057] Example three: Nitrogen-bridged bisphenol functionalized imidazolium salt {[(ArCH 2 ) 2 NCH 2 CH 2 NCHCHNR']CH}X, (X=Cl), R' is the synthesis of methyl
[0058] Under anhydrous and oxygen-free conditions, in an argon atmosphere, N,N-di(3,5-di-tert-butyl-2-hydroxybenzyl)-2-chloroethylamine (5g, 8mmol) and methylimidazole (0.8 mL, 9.6 mmol) was added to toluene, reacted at 115°C for 6 days, and the nitrogen-bridged bisphenol functionalized imidazolium salt was precipitated from the system, filtered by suction, washed with ethyl acetate, and the yield was 40%.
[0059] Carry out elemental analysis to product, test melting point, NMR analysis, the result is as follows:
[0060] Elemental analysis and melting point
[0061]
[0062] NMR data, 1 HNMR (CDCl 3 , δ, ppm) 10.65 (s, 1H, NCHN), 8.47 (s, 2H, O-H), 7.14-7.17 (d, 2H, Ph-H), 7.07 (s, 1H, NCHN), 6.98 (s, 1H , NCHC), 6.86-6.88 (d, 2H, Ph-H), 4.81-4.84 (t, 2H, NCH 2 C), 4.01(m, 1H, CH 3 ), 3.85(s, 4H, Ph CH ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com