Catalyst for hydroxylation reaction of phenol and preparation method thereof
A phenol hydroxylation and catalyst technology, applied in the field of chemical engineering, can solve problems such as corrosive environment, many by-products, and many production steps, and achieve the effects of good pore order, mild reaction conditions and high reaction activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
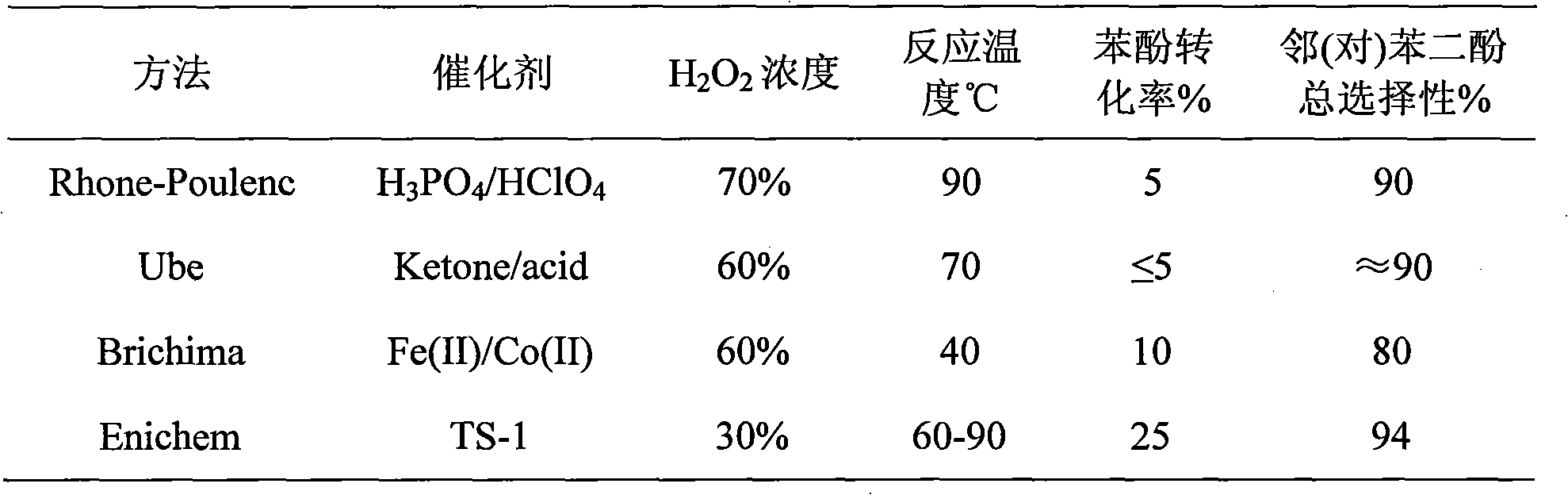
Image
Examples
Embodiment 1
[0019] Dissolve 0.1 g of tetrasulfonate iron phthalocyanine in 10 mL of deionized water, add 3.72 g of CTMAB, fully oscillate and dissolve in ultrasonic waves, and form a micellar solution for later use. Take 12.1g Na 2 SiO 3 9H 2 O was dissolved in 18.1mL deionized water, and the pH of the solution was adjusted to 10.5 with 4.0M sulfuric acid. After stirring at room temperature for 0.5 hours, the above-mentioned micellar solution was slowly poured into the sodium silicate solution with adjusted pH, and after stirring evenly, the Aged at room temperature for 1 hour, then moved into a stainless steel autoclave, and crystallized at 120°C for 24 hours. After cooling, filter with suction and wash with deionized water until the filtrate is colorless. The obtained solid was added to a mixture of 2.5 mL of concentrated hydrochloric acid and 300 mL of absolute ethanol (the concentration of HCl was 0.1 M), and heated to reflux for 6 hours. Then suction filter, wash with deionized w...
Embodiment 2
[0021] The preparation method is the same as Example 1, except that the amount of tetrasulfonate iron phthalocyanine added is 0.5 g. The prepared catalyst detects the metal content therein with an inductively coupled plasma emission spectrometer (ICP), and calculates that the loading capacity of the tetrasulfonic acid base iron phthalocyanine in the catalyst is 1.59 × 10 -5 mol / g.
Embodiment 3
[0023] The preparation method is the same as Example 1, except that the amount of tetrasulfonate iron phthalocyanine added is 0.9 g. The prepared catalyst detects the metal content therein with an inductively coupled plasma emission spectrometer (ICP), and calculates that the loading capacity of the tetrasulfonic acid base iron phthalocyanine in the catalyst is 2.15 × 10 -5 mol / g.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com