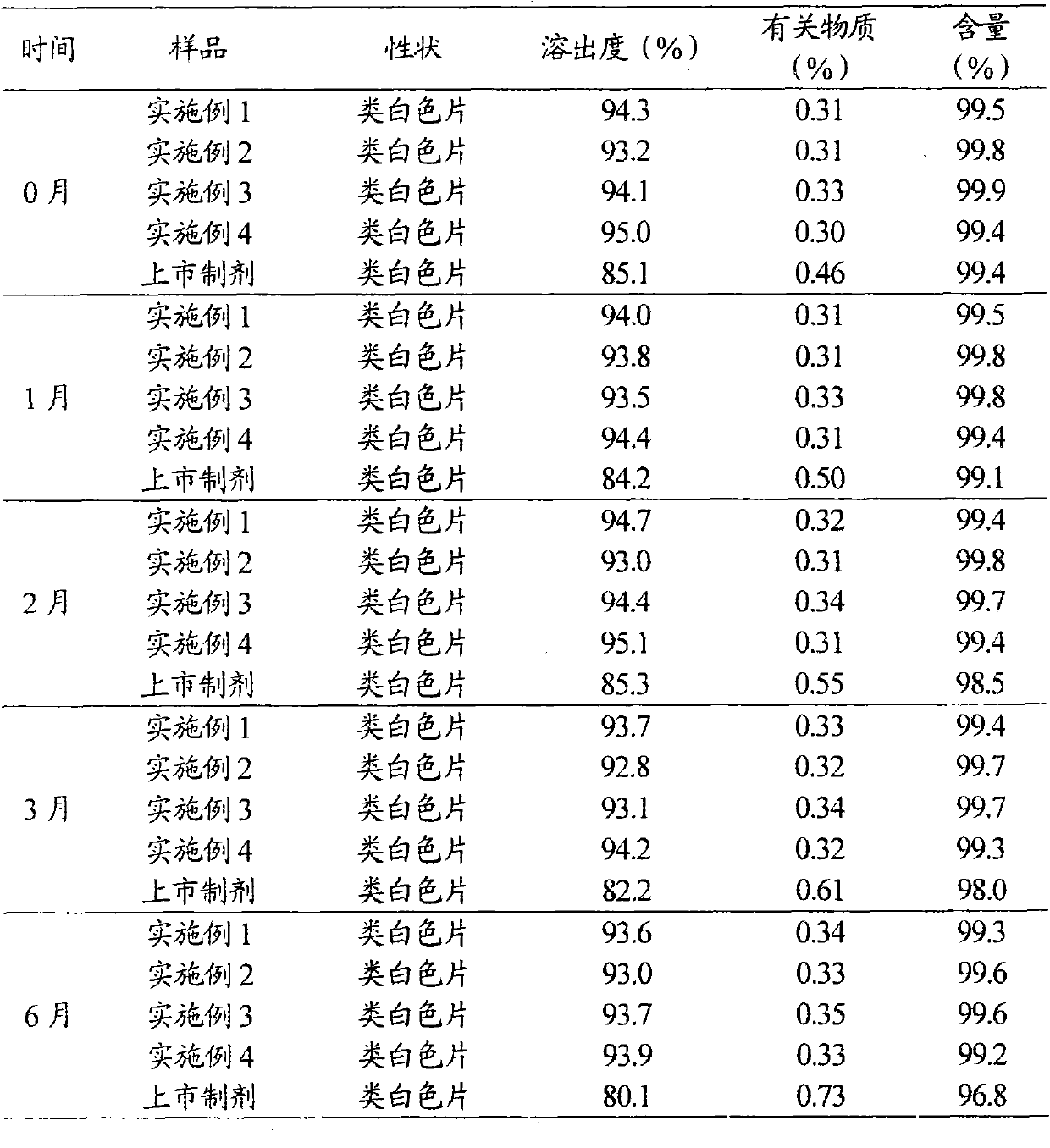
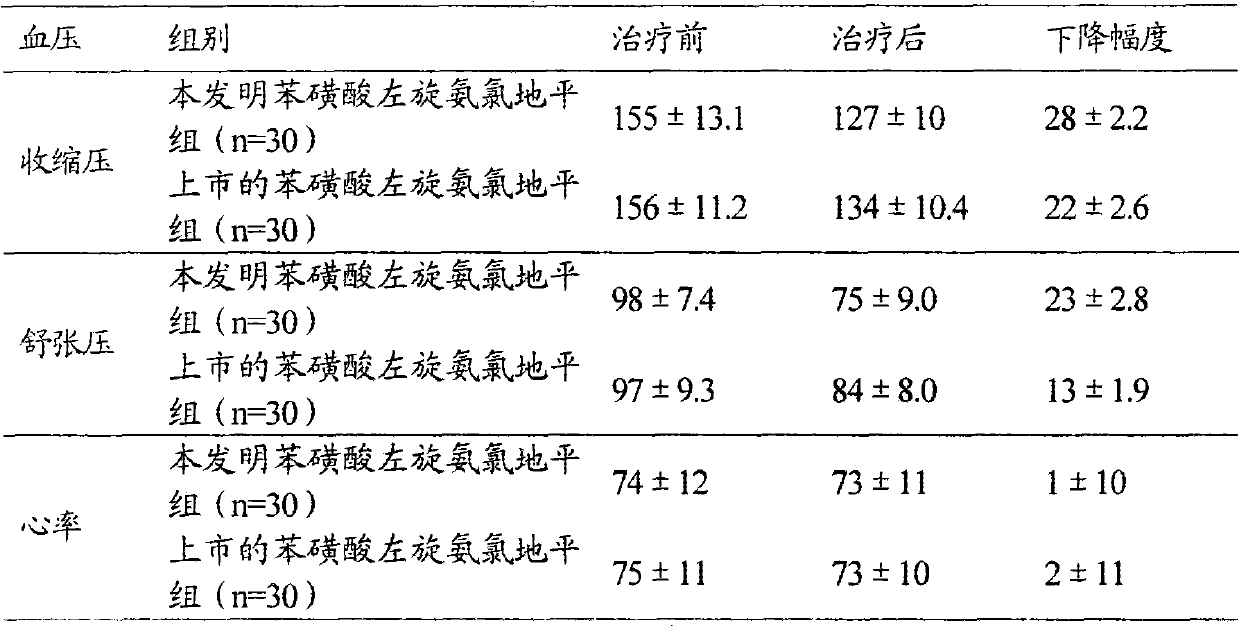
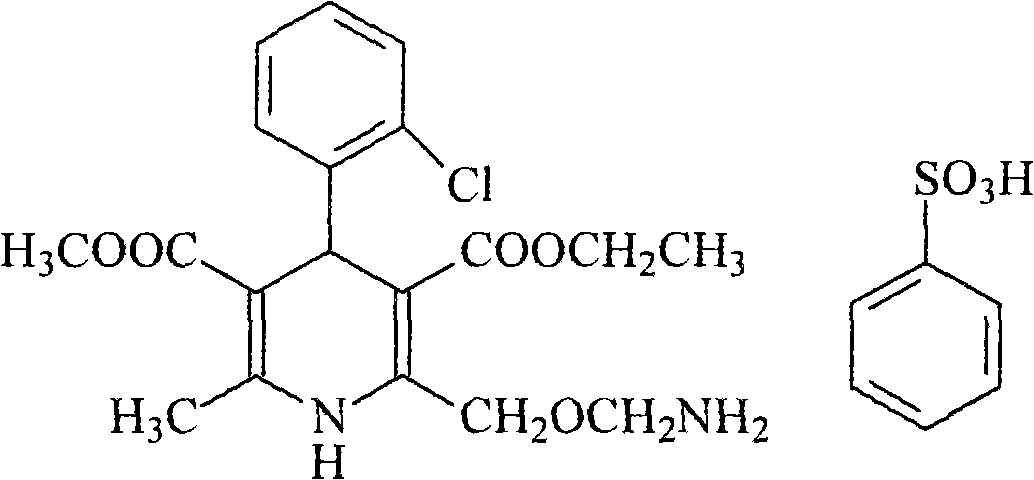
Levoamlodipine besylate liposome tablet
A technology of L-amlodipine besylate and L-besylate, which is applied in the field of liposome solid preparations, can solve the problems of poor dispersibility and absorption, poor product stability, and low bioavailability, and achieve simple excipients Inexpensive, easy to operate, high economic value effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] The preparation of embodiment 1 levamlodipine besylate liposome tablet
[0043] prescription:
[0044] Component Dosage
[0045] Levoamlodipine Besylate 2.5g
[0047] Cholesterol 1.25g
[0048] Poloxamer 188 2.5g
[0049] Starch 60g
[0050] Microcrystalline Cellulose 40g
[0051] Croscarmellose Sodium 13g
[0052] Povidone K30 5g
[0053] Micronized silica gel 5g
[0054] Magnesium Phosphatidate 2g
[0055] Preparation Process:
[0056] (1) Dissolve 10 g of egg yolk lecithin, 1.25 g of cholesterol, and 2.5 g of poloxamer 188 in 600 ml of a mixed solvent of ethanol and acetone with a volume ratio of 1:1, mix well, and remove the mixture under reduced pressure on a rotary thin film evaporator. Solvent to prepare phospholipid film;
[0057] (2) Add 300ml of potassium dihydrogen phosphate-dipotassium hydrogen phosphate buffer solution with a pH value of 6.8, adjust the pH value to 5.8 with 10% phosphoric acid solution, shake and s...
Embodiment 2
[0062] The preparation of embodiment 2 levamlodipine besylate liposome tablet
[0063] prescription:
[0064] Component Quantity
[0065] Levoamlodipine Besylate 5g
[0066] Egg yolk lecithin 50g
[0067] Cholesterol 25g
[0068] Poloxamer 188 15g
[0069] Microcrystalline Cellulose 90g
[0070] Mannitol 40g
[0071] Carboxymethyl Starch Sodium 15g
[0072] Povidone K30 10g
[0074] Micronized silica gel 10g
[0075] Preparation Process:
[0076] (1) 50g egg yolk lecithin, 25g cholesterol, and 15g poloxamer 188 are dissolved in 1500ml of ethanol and acetone mixed solvent with a volume ratio of 1: 1, mix well, remove the mixed solvent under reduced pressure on a rotary thin film evaporator, Prepare phospholipid film;
[0077] (2) Add 800ml of potassium dihydrogen phosphate-dipotassium hydrogen phosphate buffer solution with a pH value of 6.8, adjust the pH value to 7.0 with 2mol / L potassium hydroxide solution, shake and stir to completely hy...
Embodiment 3
[0083] The preparation of embodiment 3 levamlodipine besylate liposome sheet
[0084] prescription:
[0085] Component Dosage
[0086] Levoamlodipine Besylate 2.5g
[0087] Egg yolk lecithin 20g
[0088] Cholesterol 10g
[0089] Poloxamer 188 5g
[0090] Microcrystalline Cellulose 60g
[0091] Dextrin 26g
[0092] 10g pregelatinized starch
[0093] Hypromellose 4g
[0095] Stearic acid 4g
[0096] Preparation Process:
[0097] (1) 20g egg yolk lecithin, 10g cholesterol, 5g poloxamer 188 are dissolved in the dichloromethane solvent of 800ml, mix uniformly, remove mixed solvent under reduced pressure on the rotary thin film evaporator, make phospholipid film;
[0098] (2) Add 400ml of boric acid-borax buffer solution with a pH value of 6.8, adjust the pH value to 6.5 with 5% citric acid solution, shake and stir to completely hydrate the phospholipid film, and emulsify it 4 times with a high-speed tissue masher , 4min each time, rotating speed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com