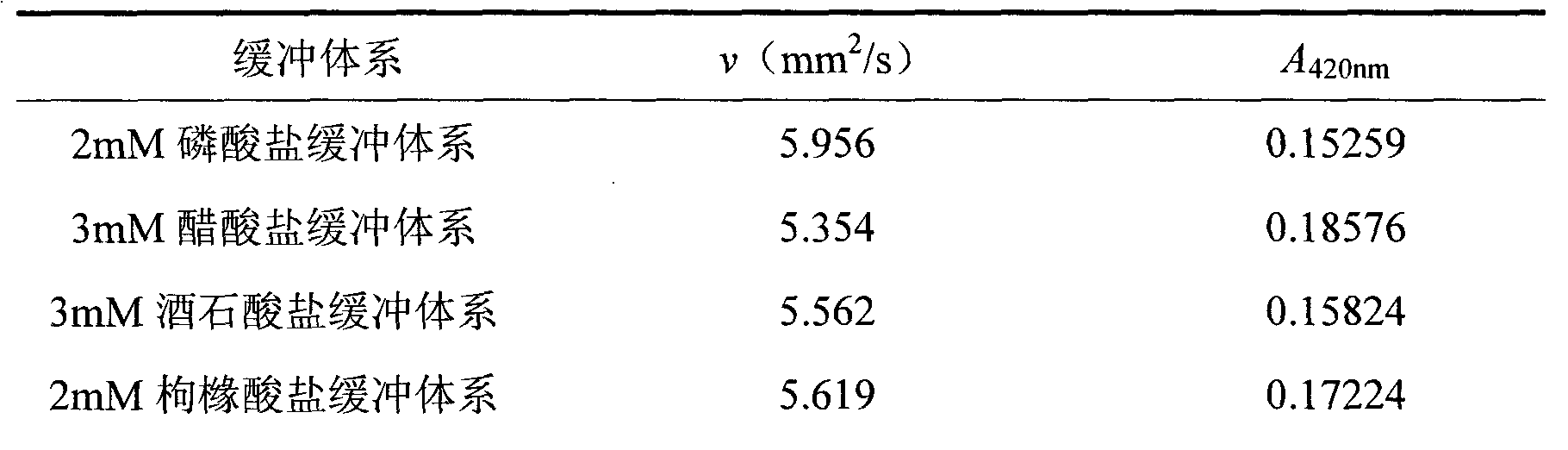Drug composite and application thereof
A composition and medicine technology, applied in the field of medicine, can solve the problems such as difficulty in exerting the slow-release effect of hyaluronic acid, unfavorable environmental protection, high viscosity of hyaluronic acid, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
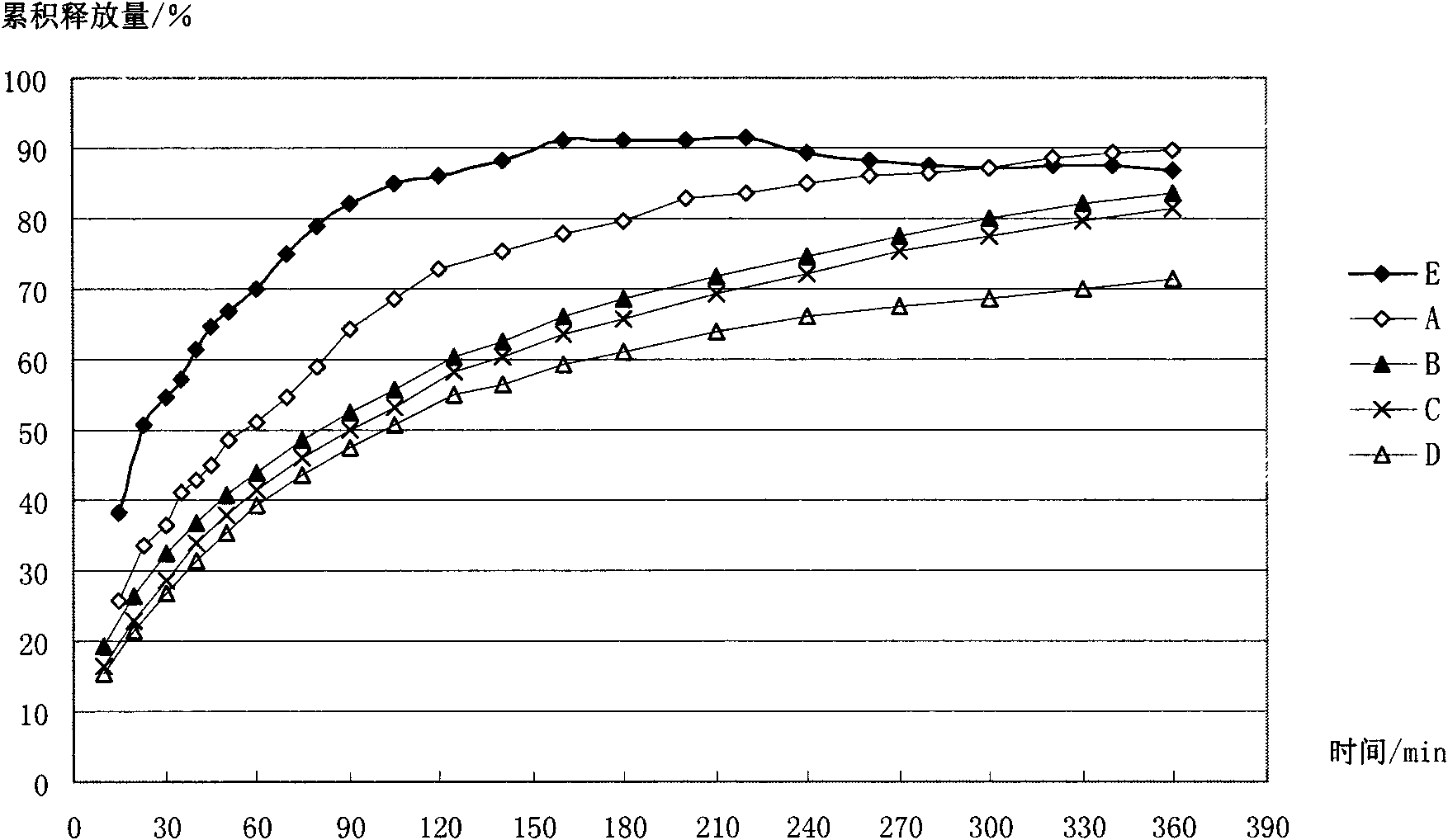
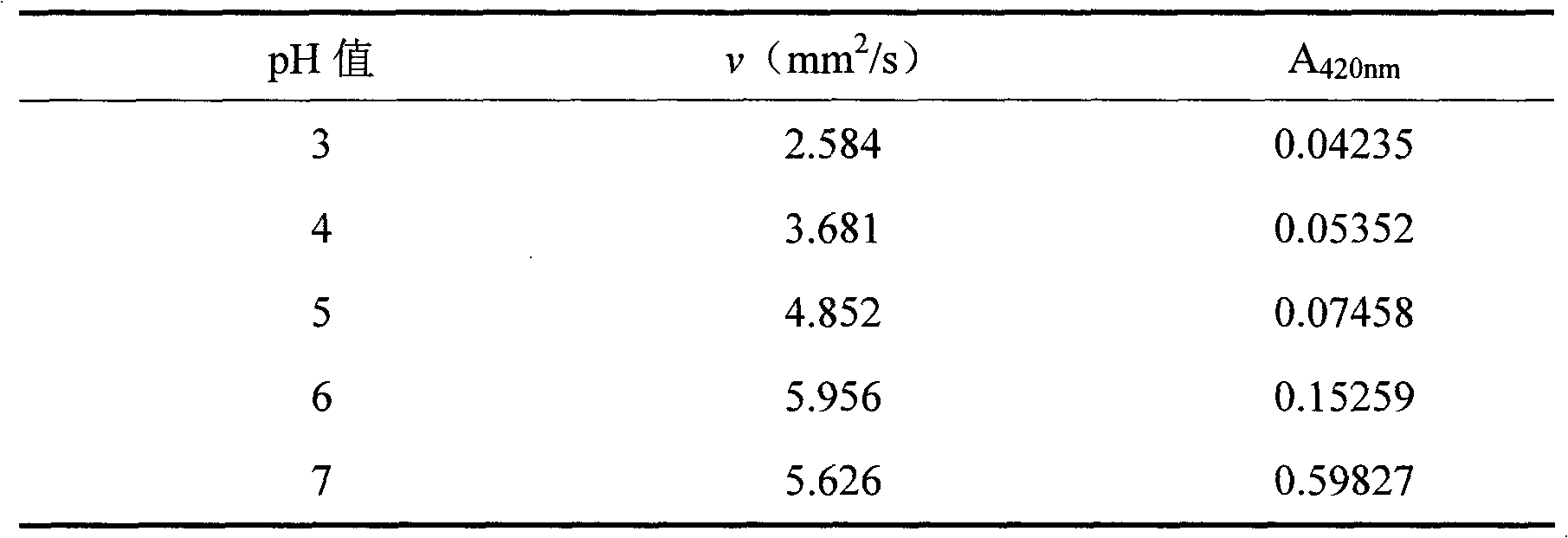
Image
Examples
Embodiment 1
[0056] The preparation of embodiment 1 injection and implant
[0057] Prescription 1-1 Sinomenine hydrochloride sodium hyaluronate injection (according to 100mL): 0.5g sinomenine hydrochloride, 2g sodium hyaluronate, 0.005g sodium dihydrogen phosphate dihydrate, 0.056 disodium hydrogen phosphate dodecahydrate g, sodium chloride 0.55g, EDTA 0.03g, sodium bisulfite 0.1g, and the balance is water for injection.
[0058] Prescription 1-2 Sinomenine hydrochloride sodium hyaluronate injection (according to 100mL): 1g sinomenine hydrochloride, 1.5g sodium hyaluronate, 0.005g sodium dihydrogen phosphate dihydrate, 0.065 disodium hydrogen phosphate dodecahydrate g, sodium chloride 0.5g, EDTA 0.03g, sodium bisulfite 0.2g, and the balance is water for injection.
[0059] Prescription 1-3 Sinomenine hydrochloride sodium hyaluronate injection (according to 100mL): 2.5g sinomenine hydrochloride, 1g sodium hyaluronate, 0.005g sodium dihydrogen phosphate dihydrate, 0.065 disodium hydrogen ph...
Embodiment 2
[0070] The preparation of embodiment 2 injection and implant
[0071]Prescription 2-1 Sinomenine hydrochloride cross-linked sodium hyaluronate gel (according to 100mL): 0.5g sinomenine hydrochloride, 2g cross-linked sodium hyaluronate, 0.005g sodium dihydrogen phosphate dihydrate, dodecahydrate phosphoric acid 0.06g of disodium hydrogen, 0.55g of sodium chloride, 0.03g of EDTA, 0.1g of sodium metabisulfite, and the balance is water for injection.
[0072] Prescription 2-2 Sinomenine hydrochloride cross-linked sodium hyaluronate gel (according to 100mL): 1g sinomenine hydrochloride, 1.5g cross-linked sodium hyaluronate, 0.005g sodium dihydrogen phosphate dihydrate, dodecahydrate phosphoric acid Disodium hydrogen 0.065g, sodium chloride 0.5g, EDTA 0.03g, sodium bisulfite 0.2g, and the balance is water for injection.
[0073] Prescription 2-3 Sinomenine hydrochloride cross-linked sodium hyaluronate gel (100mL): 1.5g sinomenine hydrochloride, 2g cross-linked sodium hyaluronate, 0...
Embodiment 3
[0089] The preparation of embodiment 3 external gel and propellant
[0090] Prescription 3-1 Sinomenine hydrochloride sodium hyaluronate gel (according to 100mL): 0.5g sinomenine hydrochloride, 1g sodium hyaluronate, 0.007g acetic acid, 0.04g sodium acetate, 3g glycerin, 0.03g EDTA, coke Sodium sulfite 0.1g, methylparaben 0.02g, propylparaben 0.01g, HPMC 2g, and the balance is purified water.
[0091] Prescription 3-2 Sinomenine hydrochloride sodium hyaluronate gel (according to 100mL): 1g sinomenine hydrochloride, 2g sodium hyaluronate, 0.03g EDTA, 0.2g sodium bisulfite, 0.03g ethyl paraben , carbomer 1.2g, triethanolamine 1g, polyethylene glycol 4005g, and the balance is purified water.
[0092] Prescription 3-3 Sinomenine hydrochloride sodium hyaluronate gel (according to 100mL): 2g sinomenine hydrochloride, 3g sodium hyaluronate, 0.08g boric acid, 0.45g sodium chloride, 0.05g EDTA, sodium bisulfite 0.2g, potassium sorbate 0.5g, and the balance is purified water.
[0093...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com