Nickel-base catalyst used for autothermal reforming of ethanol for producing hydrogen and preparation method thereof
A nickel-based catalyst, autothermal reforming technology, applied in chemical instruments and methods, metal/metal oxide/metal hydroxide catalysts, physical/chemical process catalysts, etc., can solve problems such as catalyst deactivation, and achieve hydrogen High yield, improved low-temperature reactivity, and stable structure
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
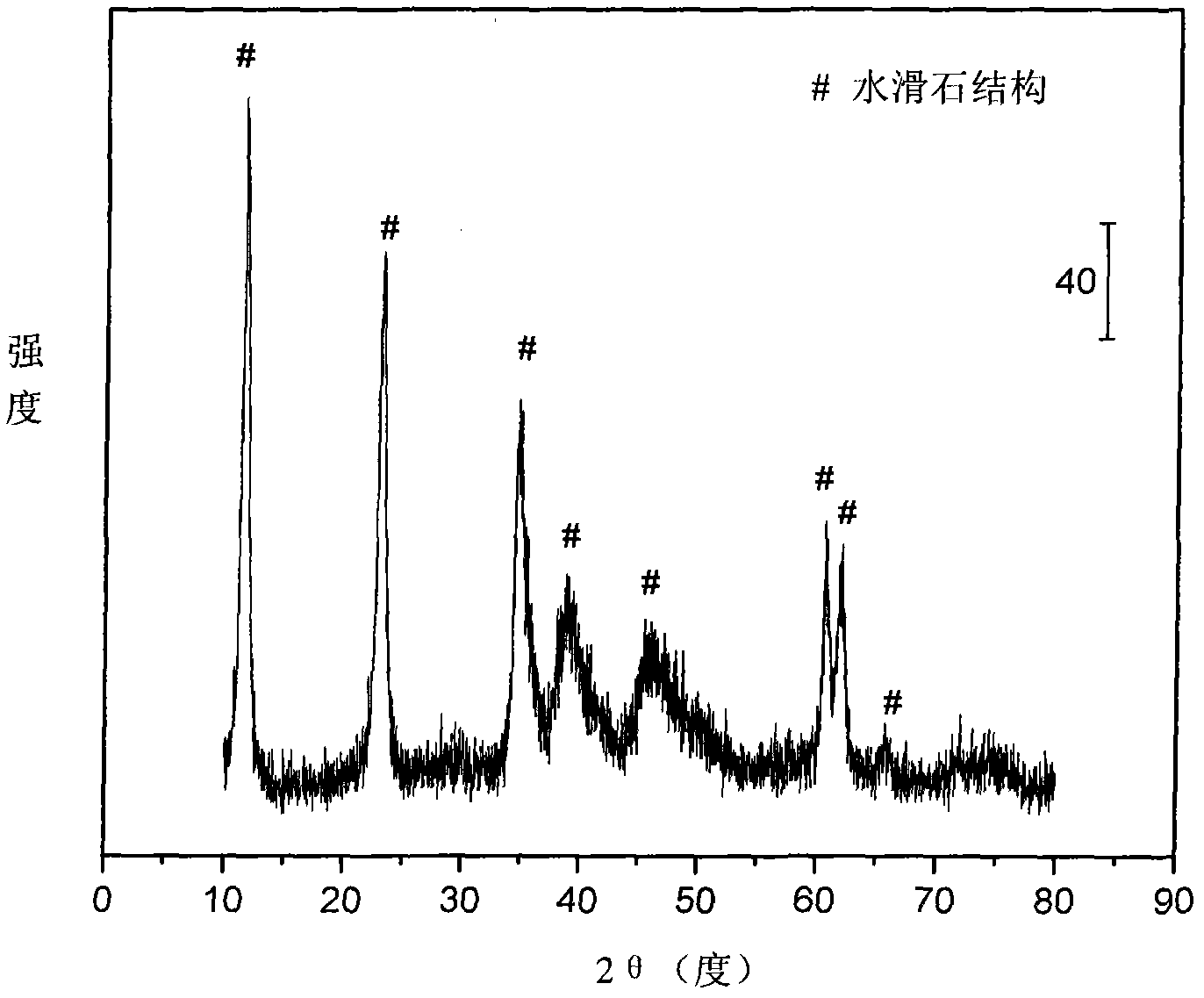
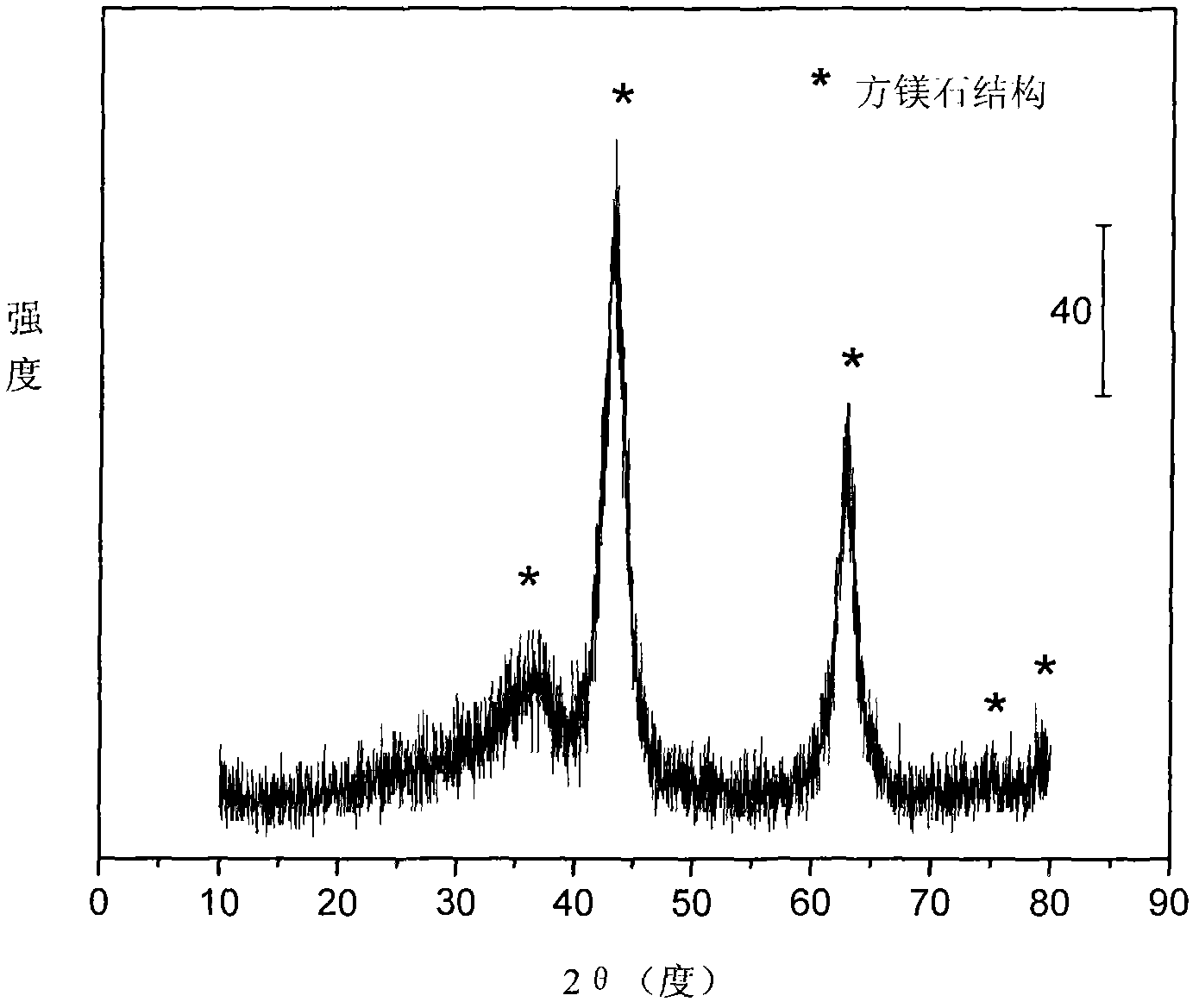
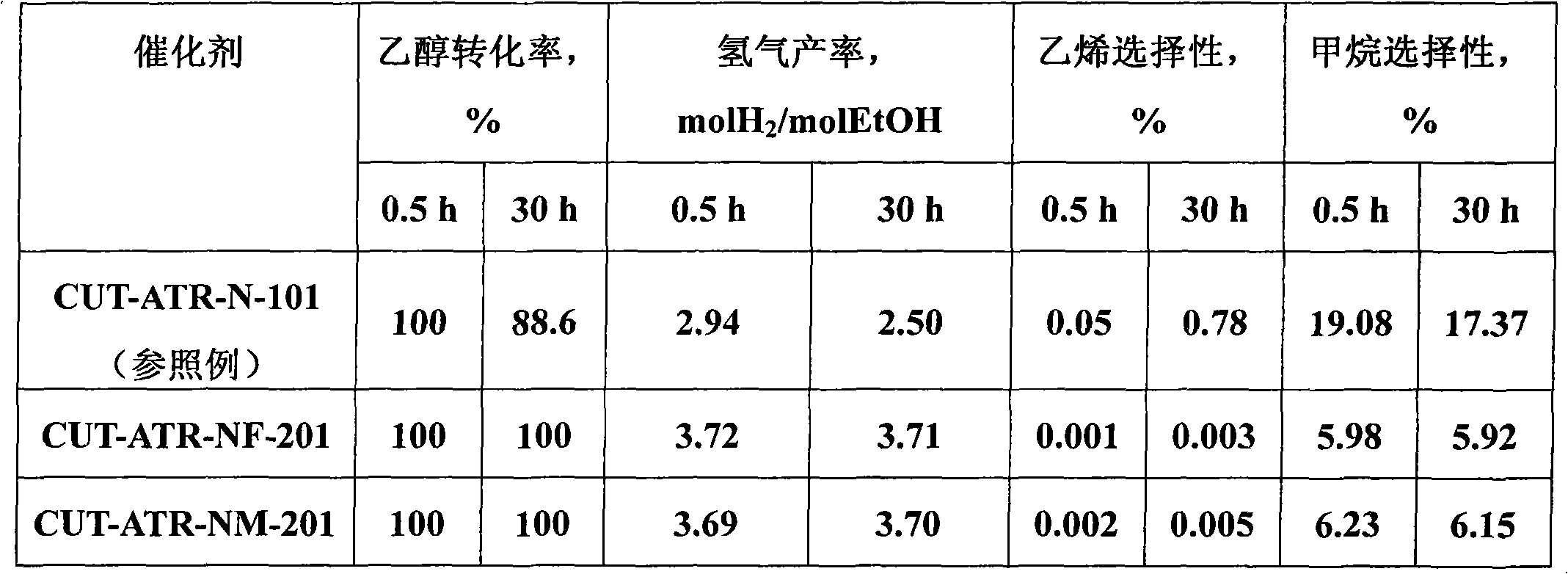
[0031] Weigh 4.410 grams of Ni (NO 3 ) 2 ·6H 2 O, 27.402 grams of Mg (NO 3 ) 2 ·6H 2 O, 7.564 grams of Al(NO 3 ) 3 9H 2 O and 8.146 g of Fe(NO 3 ) 3 9H 2 O, add 161ml of deionized water to make solution #1. Weigh 12.905 grams of NaOH and 2.137 grams of NaOH 2 CO 3 , was added to 343ml of deionized water to prepare solution #2. Subsequent steps were the same as those in Reference Example 1 to obtain catalyst CUT-ATR-NF-201. The weight composition of the catalyst is as follows: the content of nickel oxide is 13.2%, the content of magnesium oxide is 53.8%, the content of aluminum oxide is 12.8%, and the content of iron oxide is 21.2%.
[0032] The results of the catalyst in the ethanol autothermal reforming reaction are shown in Table 1. The reaction conditions are temperature 550°C, water / ethanol / oxygen molar ratio 3 / 1 / 0.5, normal pressure, and space velocity 11000h -1 , its ethanol conversion was stable at 100%, and its hydrogen yield was stable at about 3.7molH ...
Embodiment 2
[0034] Weigh 4.227 grams of Ni (NO 3 ) 2 ·6H 2 O, 28.223 grams of Mg(NO 3 ) 2 ·6H 2 O and 10.907 g of Al(NO 3 ) 3 9H 2 O, measure the weight ratio of 4.460ml and be 50% Mn (NO 3 ) 2 Solution, add 166ml of deionized water to make solution #1. Weigh 13.292 grams of NaOH and 2.201 grams of NaOH 2 CO 3 , was added to 353ml of deionized water to prepare solution #2. Subsequent steps were the same as in Reference Example 1 to obtain catalyst CUT-ATR-NM-201. The weight composition of the catalyst is as follows: the content of nickel oxide is 13.4%, the content of magnesium oxide is 54.9%, the content of aluminum oxide is 18.3%, and the content of manganese dioxide is 13.4%.
[0035] The results of the catalyst in the ethanol autothermal reforming reaction are shown in Table 1. The reaction conditions are temperature 550°C, water / ethanol / oxygen molar ratio 3 / 1 / 0.5, normal pressure, and space velocity 11000h -1 , the ethanol conversion rate was 100%, and the hydrogen prod...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com