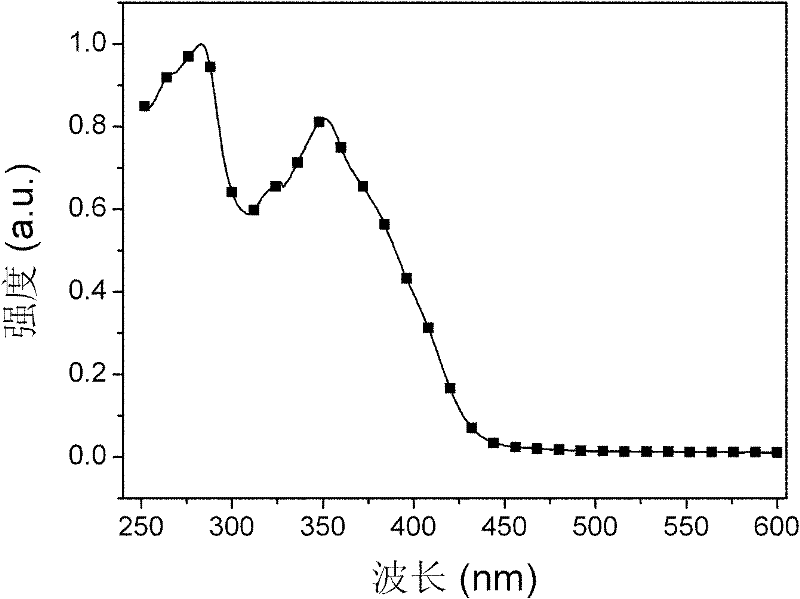
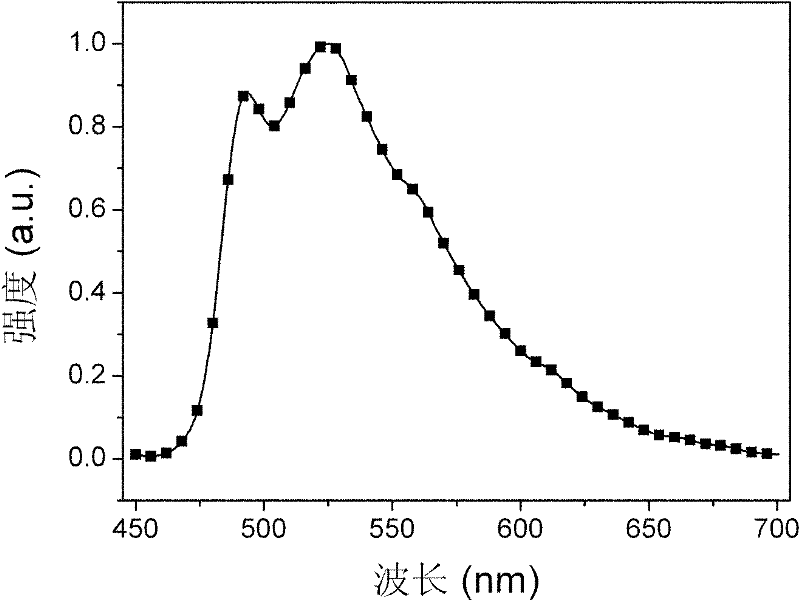
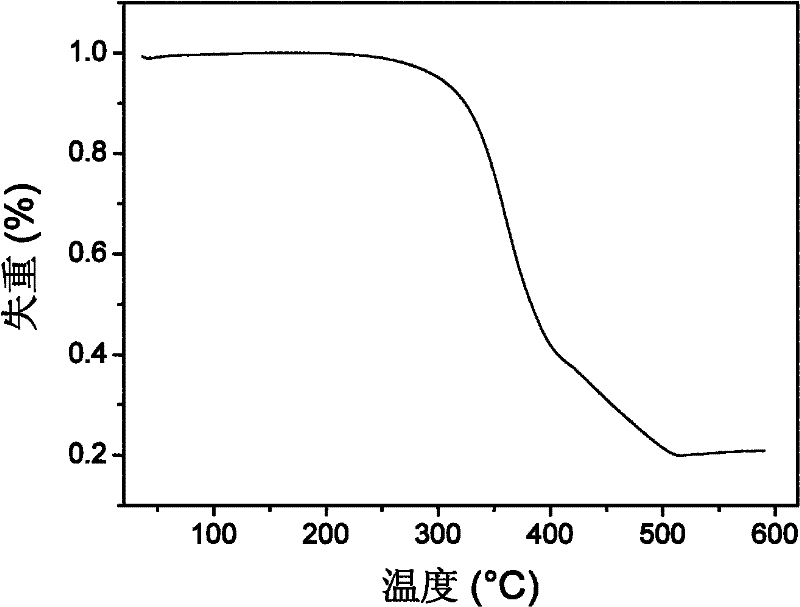
Bi(phenylpyridine) fluorene derivatives and binuclear cyclometalated platinum complex liquid crystal polarized luminescent materials
A platinum complex, pyridine phenyl technology, applied in the field of liquid crystal polarized organic light-emitting materials, can solve the problem of low luminous efficiency and luminous brightness of liquid crystal polarized OLEDs, restricting the development and application of liquid crystal polarized light emitting diodes, unfavorable high-efficiency, high-brightness liquid crystals problems such as backlight source, to achieve the effect of easy synthesis and purification, easy product, good liquid crystal performance and polarized luminescence performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] Synthesis of 2-(4'-bromophenyl)-5-(alkoxymethyl)pyridine
[0048]
[0049] 1.1 Synthesis of 2-(4'-bromophenyl)-5-(butoxymethyl)pyridine
[0050] In a 100mL three-necked flask, add 5.0g (25mmol) 4-bromophenylboronic acid ester, 6.1g (25mmol) 2-bromo-5-(butoxymethyl)pyridine, 0.58g (0.5mmol) Pd(PPh 3 ) 4 , 15mL 2M K 2 CO 3 Solution, 30mL of toluene and 15mL of ethanol were reacted at 80°C for 24h under the protection of nitrogen. After cooling, the reaction solution was poured into saturated NH 4 Cl solution, extracted with dichloromethane, the extract was washed with water, anhydrous Na2SO 4 After drying, distilling off the solvent, and column separation (petroleum ether / ethyl acetate=20:1), 6.8 g of white solid was obtained. Yield 85.6%. 1 H NMR (400MHz, CDCl 3 , TMS), 8.65 (s, 1H), 7.90-7.89 (d, J=8.4Hz, 2H), 7.78-7.76 (d, J=7.0Hz, 1H), 7.71-7.69 (d, J=8.1Hz, 1H), 7.62-7.60(d, J=8.4Hz, 2H), 4.57(s, 2H), 4.06-4.00(t, 2H), 3.55-3.52(t, 2H), 1.70-1.60(m, 2H) ...
Embodiment 2
[0056] Synthesis of 5-(alkoxymethyl)-2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pyridine
[0057]
[0058] 2.1 Synthesis of 5-(butoxymethyl)-2-(4-(4,4,5,5-tetramethyl-1,3,2-dioxaborolan-2-yl)phenyl)pyridine
[0059] In a 100mL three-necked flask, add 2.0g (5.4mmol) 2-(4'-bromophenyl)-5-(butoxymethyl)pyridine, 1.5g (5.9mmol) bisanalyl borate, 1.6g (16.2mmol) potassium acetate, 0.13g (0.16mmol) PdCl 2 (dppf)CH 2 Cl 2 And 60mL of dimethyl sulfoxide, reacted at 80°C for 24h under the protection of nitrogen. Cool, pour the reaction solution into 100mL water, extract with dichloromethane, wash the extract with water, anhydrous Na 2 SO 4 Drying, distillation to remove the solvent, and column separation (petroleum ether / ethyl acetate=10:1) yielded 1.2 g of off-white solid with a yield of 60.5%. 1 H NMR (400MHz, CDCl 3 , TMS), δ (ppm): 8.67 (s, 1H), 8.03-8.01 (d, J = 8.0Hz, 2H), 7.94-7.92 (d, J = 8.0Hz, 2H), 7.78 (s, 2H) , 4.57(s, 2H), 3.54-3.50(t, 2H), 1.67-1....
Embodiment 3
[0065] Synthesis of 2,7-bis[4'-(4"-alkoxymethyl-2"-pyridine)phenyl]-9,9-bis(dodecyl)fluorene
[0066]
[0067] 3.1 Synthesis of 2,7-bis[4′-(4″-butoxymethyl-2″-pyridine)phenyl]-9,9-bis(dodecyl)fluorene
[0068] In a 100mL three-necked flask, add 1.0g (2.7mmol) 2-[(4′-bromophenyl)-5-(alkoxymethyl)pyridine]-4,4,5,5-tetramethyl-1, 3,2-Dioxaborane, 0.8g (1.2mmol) 9,9-dioctyl-2,7-dibromofluorene, 93mg (0.08mmol) Pd(PPh 3 ) 4 , 15mL 2M K 2 CO 3 Solution, 30mL of toluene and 15mL of ethanol were reacted at 80°C for 24h under the protection of nitrogen. After cooling, the reaction solution was poured into saturated NH 4 Cl solution, extracted with dichloromethane, the extract was washed with water, anhydrous Na 2 SO 4 After drying, distilling off the solvent, and column separation (petroleum ether / ethyl acetate=10:1), 0.89 g of white solid was obtained after drying, with a yield of 75.6%. 1 H NMR (400MHz, CDCl 3 , TMS), 8.70(s, 2H), 8.15-8.13(d, J=8.2Hz, 4H), 7.83-7.81(m, 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thermal decomposition temperature | aaaaa | aaaaa |
| internal quantum efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com