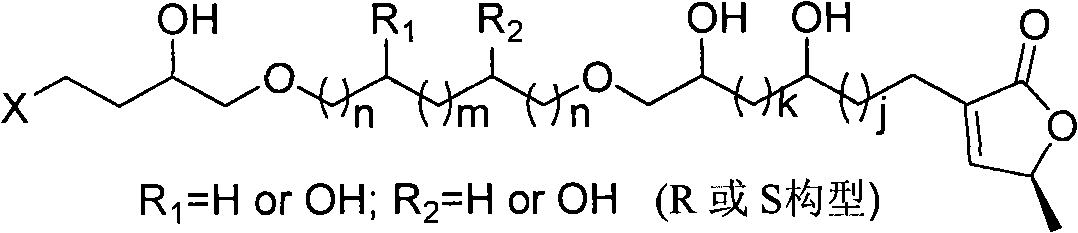
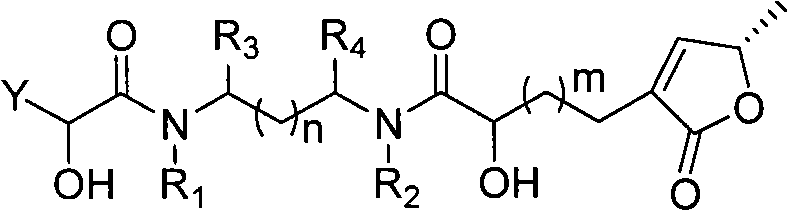
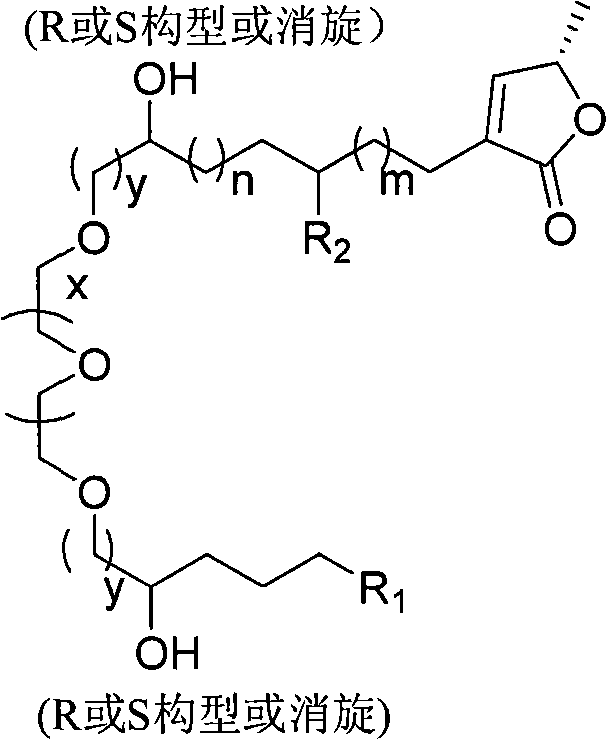
Annonaceous acetogenin compound and synthesis method and application thereof
A technology of annua lactone and a compound, which is applied in the simplified field of annua lactone compound and its synthesis, can solve the problems of not obtaining a compound, restricting research on finished drugs, etc., and achieves high anticancer activity and selectivity, and low-cost and easy-to-use raw materials. The effect of obtaining and preparation method is simple
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044]
[0045] Take 0.07moL of compound 20 in a single-necked bottle, add 1.6g of benzyltriethylammonium chloride, 0.084moL of compound 21, stir at room temperature for 3min, slowly add 55mL of 50% sodium hydroxide solution, and stir vigorously at room temperature for 3.5h , add 40mL water to quench the reaction, the reaction solution is extracted with 40mL×3 ether, the organic phases are combined and washed with saturated ammonium chloride and sodium chloride solution successively, dried over anhydrous sodium sulfate, concentrated and column chromatography to obtain a colorless liquid 22 (11.7 g, 80%).
[0046] [α] 25 D : 6.1 (c 2.6, CHCl 3 ).
[0047] 1 H NMR (400MHz, CDCl 3 ): δ7.35-7.27(m, 5H), 4.58(s, 2H), 3.80(dd, J=11.6, 3.0Hz, 1H), 3.75-3.63(m, 4H), 3.45(dd, J=11.6 , 5.8Hz, 1H), 3.18-3.16(m, 1H), 2.80-2.78(m, 1H), 2.62(dd, J=5.0, 2.7Hz, 1H) ppm; HRMS (ESI): C 12 h 16 o 3 calculated[M+H] + 209.1172, found 209.1175, [M+Na] + 231.0992, found 231.0988.
Embodiment 2
[0049]
[0050] At -78°C under the protection of argon, slowly add 13 mmol of n-BuLi (2.5M in hexane) dropwise to 13 mmol of compound 23 in 20 mL of anhydrous tetrahydrofuran, react for 45 minutes, and slowly add 13 mmol of BF 3 ·Et 2 O, after reacting for 30 minutes, slowly add 6.5 mmol of compound 22 in 5 mL of anhydrous tetrahydrofuran solution, continue to stir for 3 h, quench the reaction with 5 mL of saturated ammonium chloride solution, spin off tetrahydrofuran under reduced pressure, and extract with 15 mL×3 diethyl ether, The organic phases were combined and washed successively with saturated sodium bicarbonate and saturated sodium chloride solution, dried over anhydrous sodium sulfate, and the intermediate obtained by concentration was dissolved in 20 mL of anhydrous dichloromethane, and 52 mmol of diisopropyl Ethylamine, after cooling to 0°C, slowly add 32.5mmol of MOMCl, stir the reaction at room temperature for 20h, quench the reaction with 5mL of saturated amm...
Embodiment 3
[0055]
[0056] Dissolve 4.26mmol of intermediate 24 in 20ml of absolute ethanol, add 0.302g (20%m) of 10% Pd / C, 2.0mL of glacial acetic acid, react for 16h under the action of hydrogen balloon, and filter with diatomaceous earth , washed with ethanol, concentrated to obtain the intermediate, added 5.11mmol of 21 at room temperature, 0.18g of benzyltriethylammonium chloride, slowly added 3.5mL of 50% NaOH aqueous solution, stirred at room temperature for 22h, diluted with 3mL of water, 15 mL×3 of diethyl ether was extracted, the organic phases were combined and washed successively with saturated ammonium chloride and sodium chloride, dried over anhydrous sodium sulfate, concentrated and column chromatographed to obtain light yellow liquid compound 25 (0.99 g, 72%).
[0057] [α] 25 D =-1.8 (C=1.11, CHCl 3 );
[0058] 1 H NMR (400MHz, CDCl 3 ): δ7.28-7.15(m, 5H), 4.76(d, J=6.8Hz, 1H), 4.64(d, J=6.8Hz, 1H), 3.77(dd, J=11.7, 2.8Hz, 1H) , 3.74-3.38(m, 8H), 3.36(s, 3H), 3.1...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com