Method for preparing multilayer alpha-Ni(OH)2 or NiO nanocrystal by microwave solvothermal method
A microwave solvothermal, multi-level technology, applied in the field of nanomaterials, can solve the problems of difficult to control nanostructure, large product size, difficult synthesis, etc., and achieve the effects of easy industrial production, narrow pore size distribution, and simple production equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
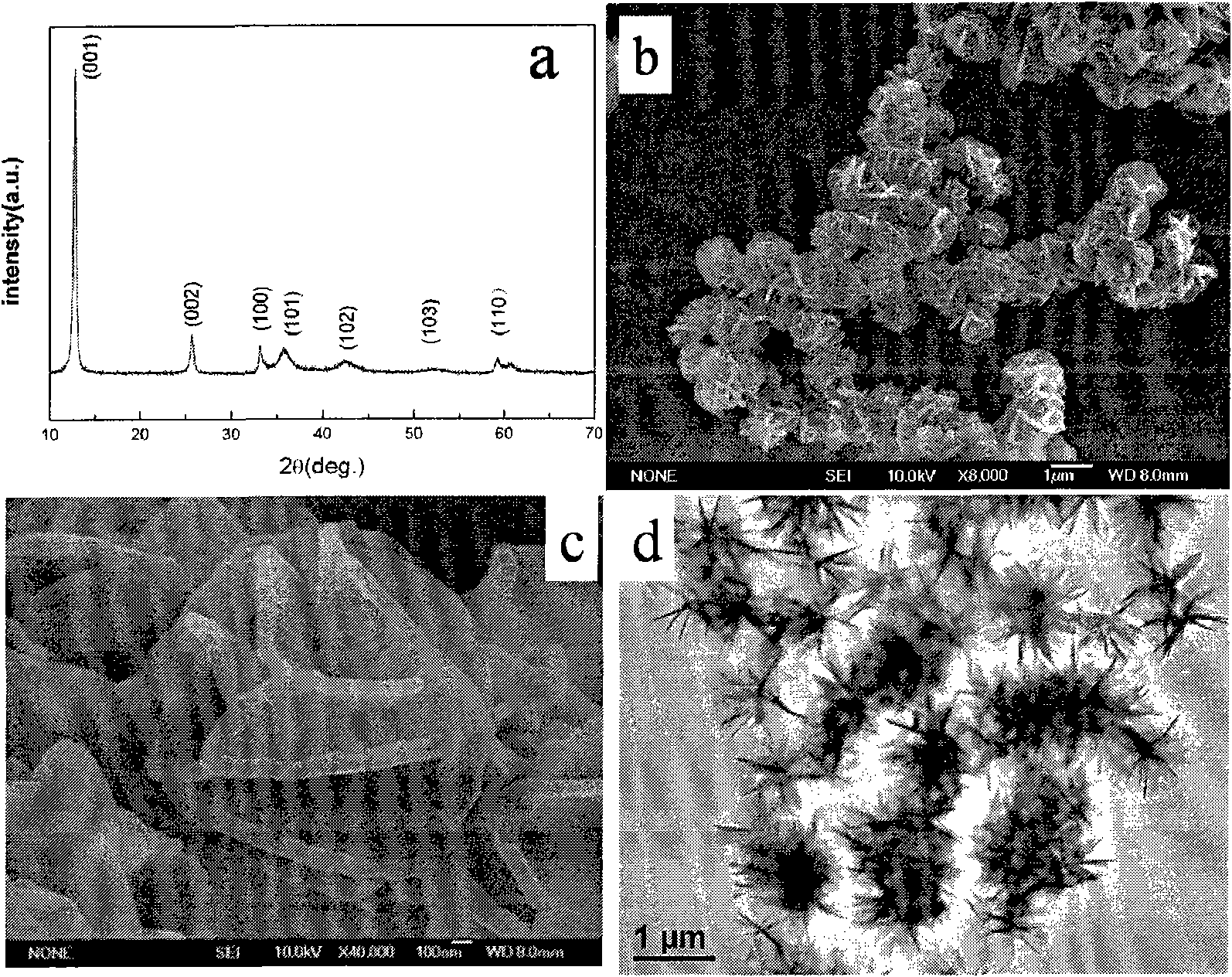
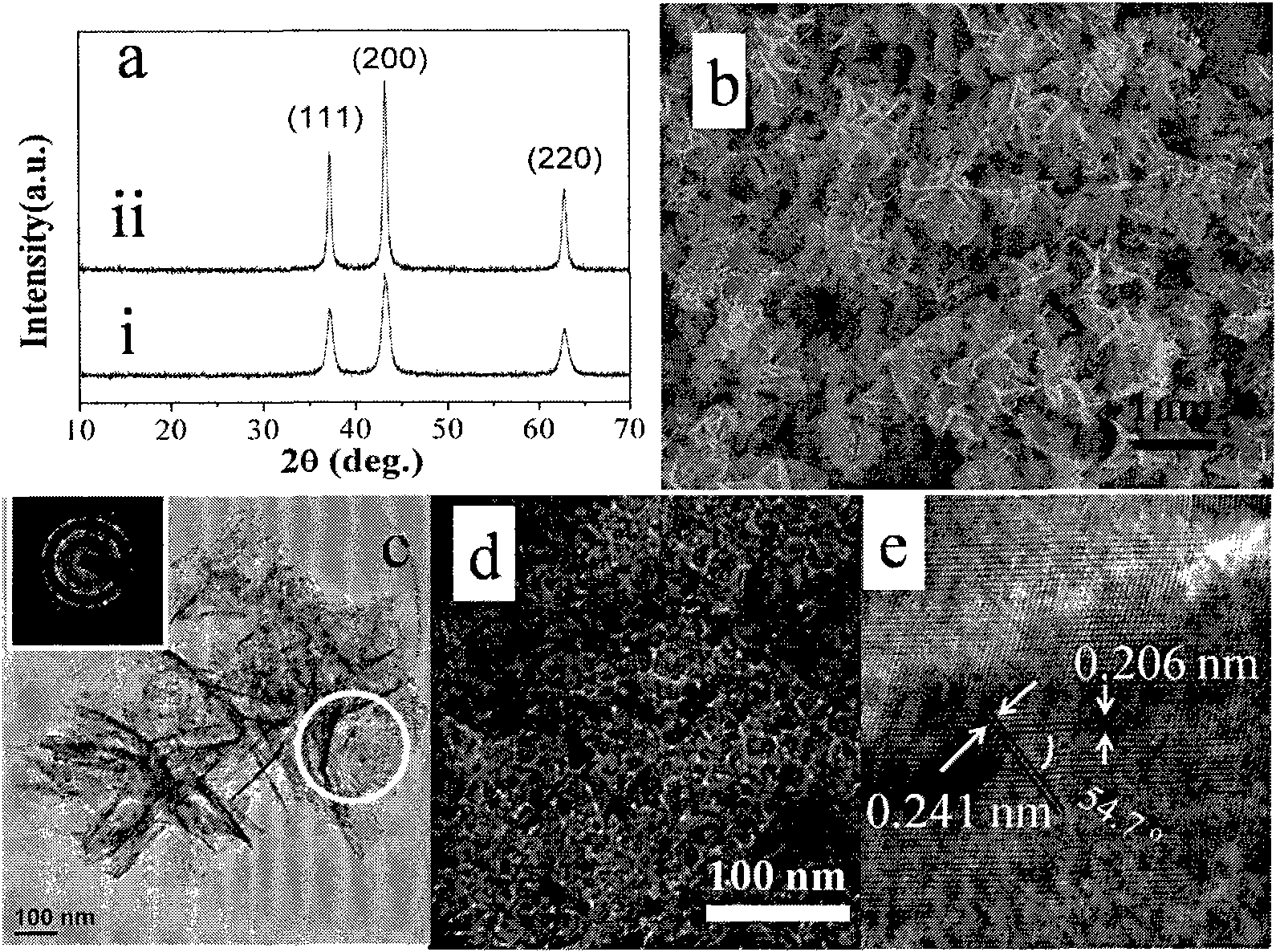
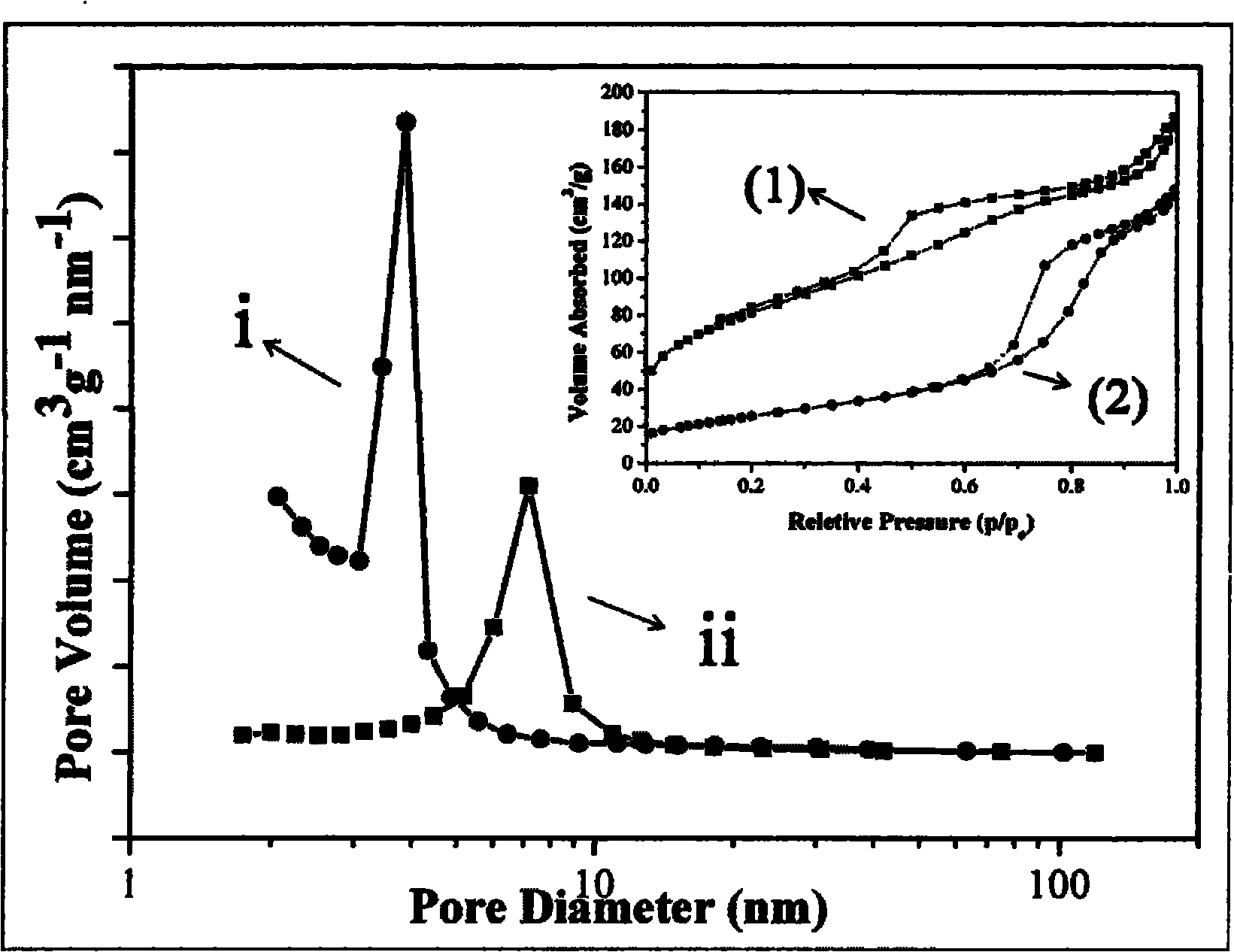
[0030] 20mmol Ni(NO 3 ) 2 ·6H 2 O was dissolved in 30 mL of anhydrous ethanol solution (Ni 2+ The concentration is 0.67mol L -1 ), magnetic stirring for 15min, rotating speed 200, make it fully dissolved, then transfer the clear precursor liquid to 50mL Teflon microwave heating container, carry out microwave heating (heating rate 25 ℃ min -1 ), and kept at 150 °C for 15 min, and cooled naturally. High-speed centrifugation (6000 rpm) to separate the green precipitate, washed three times with deionized water and absolute ethanol, and then vacuum-dried at 60 °C for 5 h to obtain green α-Ni(OH) 2 . (XRD see figure 1 (a)). The appearance of the product is flower-like, consisting of stretched flaky petals, the thickness is about 70-100 nm, the particle size is uniform, about 1 μm, and the dispersibility is good. ( figure 1 (b)-(d))
Embodiment 2
[0032] 200mmol Ni(NO 3 ) 2 ·6H 2 O was dissolved in 30 mL of anhydrous ethanol solution (Ni 2+ The concentration is 6.7mol L -1 ), magnetic stirring for 15min, rotating speed 200, make it fully dissolved, then transfer the clear precursor liquid to 50mL Teflon microwave heating container, carry out microwave heating (heating rate 25 ℃ min -1 ), and kept at 150 °C for 15 min, and cooled naturally. High-speed centrifugation (6000 rpm) to separate the green precipitate, washed three times with deionized water and absolute ethanol, and then vacuum-dried at 60 °C for 5 h to obtain green α-Ni(OH) 2 . The product phase and micromorphological characterization results are the same as in Example 1.
Embodiment 3
[0034] 2mmol Ni(NO 3 ) 2 ·6H 2 O was dissolved in 30 mL of anhydrous ethanol solution (Ni 2+ The concentration is 0.067mol L -1 ), magnetic stirring for 15min, rotating speed 200, make it fully dissolved, then transfer the clear precursor liquid to 50mL Teflon microwave heating container, carry out microwave heating (heating rate 25 ℃ min -1 ), and kept at 150 °C for 15 min, and cooled naturally. High-speed centrifugation (6000 rpm) to separate the green precipitate, washed three times with deionized water and absolute ethanol, and then vacuum-dried at 60 °C for 5 h to obtain green α-Ni(OH) 2 . The results of product phase and micromorphological characterization are the same as those of Example 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com