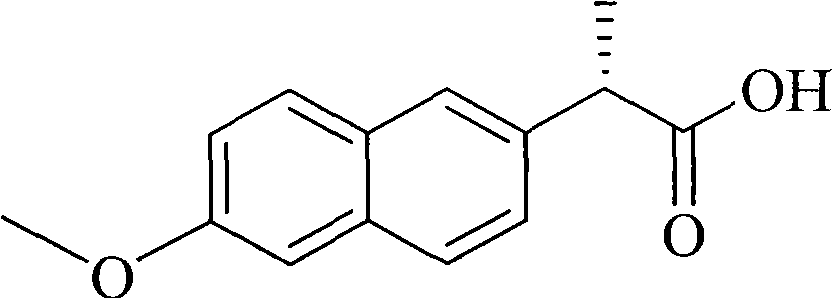
Naproxen orally-taken micro-emulsion preparation and preparation method thereof
A technology of emulsion preparation and naproxen, which is applied in anti-inflammatory agents, emulsion delivery, non-central analgesics, etc., can solve the problems of unstable drug content, cumbersome preparation process, and short biological half-life, so as to improve bioavailability High degree, broad application prospects, and simple production process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Take 1.8g of naproxen, 8.0g of medium-chain triglycerides, 30.0g of emulsifier Tween 8030.0g and 10.0g of co-emulsifier polyethylene glycol-400 (PEG-400) and heat to 25°C, stir and mix to obtain the oil phase ; Measure 55.0ml of water for injection, 66.7mg of sodium sulfite, and 69.0mg of xylitol, stir to dissolve, and heat to 25°C to obtain an aqueous phase. Stir and mix the oil phase and the water phase at a temperature of 25° C., filter to obtain a microemulsion, fill, stopper, cap, and sterilize at 121° C. for 15 minutes to obtain naproxen oral microemulsion. The average particle diameter of the microemulsion is (38.6±2.0) nm, the content of naproxen is 1.77% (W / V), and other indexes meet the requirements of pharmaceutical oral microemulsion.
Embodiment 2
[0032] Take 1.0g of naproxen, 5.0g of medium-chain triglycerides, 2.5g of emulsifier Tween 802.5g and 7.5g of 1,2-propanediol and heat to 25°C and stir to obtain an oil phase; measure 70.0ml of water for injection, sodium sulfite 69.6 mg and 87.00 mg of aspartose were stirred to dissolve, and heated to 25°C to obtain an aqueous phase. Stir and mix the oil phase and the water phase at a temperature of 25° C., filter to obtain a microemulsion, fill, stopper, cap, and sterilize at 121° C. for 15 minutes to obtain naproxen oral microemulsion. The average particle size of the microemulsion is (19.0±2.1) nm, the content of naproxen is 0.99% (W / V), and other indexes meet the requirements of pharmaceutical oral microemulsion.
Embodiment 3
[0034] Take 1.0 g of naproxen, 6.0 g of macrogol glycerol oleate (Labrafil M1944CS), 16.0 g of emulsifier polyoxyethylene castor oil (CremophorEL) and 8.0 g of co-emulsifier 1,2-propylene glycol and heat to 25° C. Mix well to obtain an oil phase; measure 75.0 ml of water for injection, 75.0 mg of sodium metabisulfite, and 80.0 mg of xylitol, stir to dissolve, and heat to 25°C to obtain an aqueous phase. Stir and mix the oil phase and the water phase at a temperature of 25° C., filter to obtain a microemulsion, fill, stopper, cap, and sterilize at 121° C. for 15 minutes to obtain naproxen oral microemulsion. The average particle diameter of the microemulsion is (29.8±1.5) nm, the content of naproxen is 0.96% (W / V), and other indexes meet the requirements of pharmaceutical oral microemulsion.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com