Di-fluoro containing compounds as cysteine protease inhibitors
Technology of a compound, amino group, in the field of difluorinated compounds as cysteine protease inhibitors
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
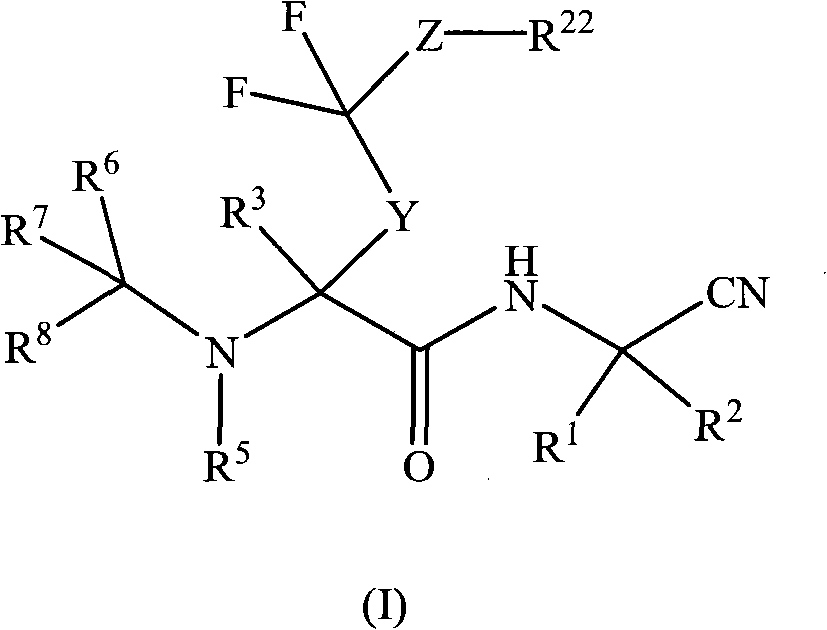
[0122] In a particular aspect, the present invention relates to a compound of formula (I) or a pharmaceutically acceptable salt thereof, wherein:
[0123] R 1 Is hydrogen or alkyl;
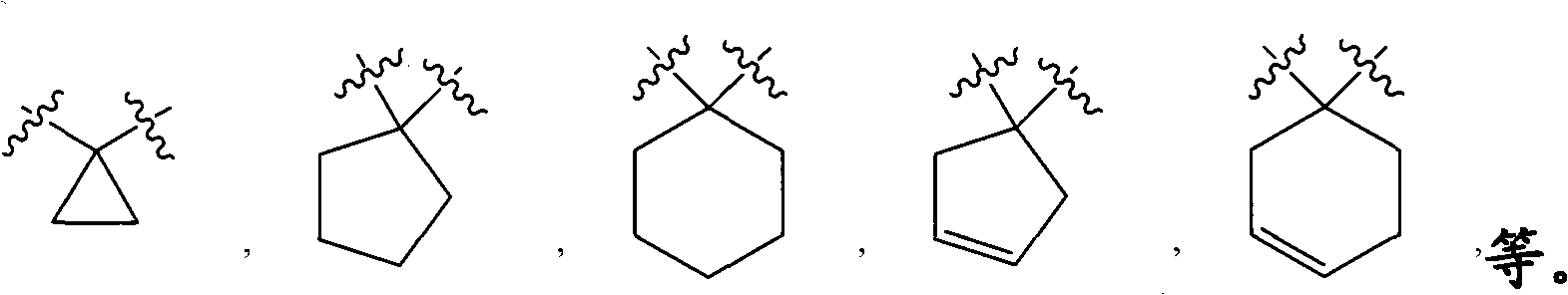
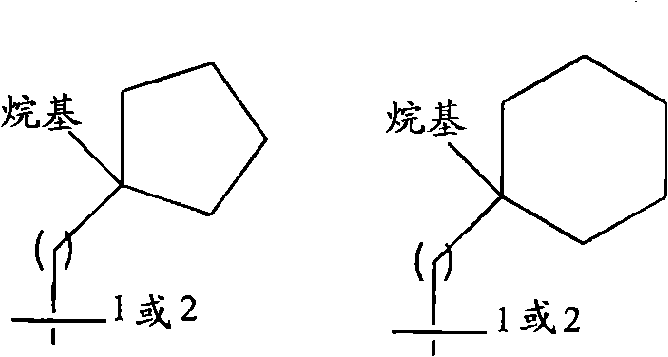
[0124] R 2 Is hydrogen, alkyl, haloalkyl, carboxyalkyl, alkoxycarbonylalkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heteroaryl, heteroaralkyl, heterocyclyl, Heterocyclylalkyl, cyano or -alkylene-XR 9 (Where X is -O-, -NR 10 -, -CONR 11 -, -S(O) n1 -, -NR 12 CO-, -CO- or -C(O)O-, where n1 is 0-2, and R 9 , R 10 , R 11 And R 12 Independently hydrogen, alkyl, haloalkyl, cycloalkyl, cycloalkylalkyl, aryl, aralkyl, heteroaryl or heteroaralkyl), wherein R 2 The aromatic ring or alicyclic ring in is optionally divided by one, two or three R a Replace, the R a Independently selected from alkyl, haloalkyl, alkoxy, hydroxy, haloalkoxy, halogen, carboxy, alkoxycarbonyl, amino, monosubstituted amino, disubstituted amino, nitro, aryloxy, benzyloxy, Acyl or arylsulfonyl, and further wherein R a The aromatic rin...
Embodiment 1
[0217] Synthesis Example 1-Scheme 1
[0218]
[0219] Scheme 1, Step 1: Synthesis of 1-aminocyclopropane nitrile hydrochloride (1-aminocyclopropane nitrile hydrochloride is commercially available)
[0220]
[0221] At room temperature and nitrogen, in a 2L Erlenmeyer flask, benzophenone imine (25g, 0.138mol, Aldrich) and aminoacetonitrile hydrochloride (25g, 0.270mol, Lancaster) in dichloromethane (1000mL) The mixture was stirred for five days. The reaction mixture was filtered to remove the precipitated ammonium chloride, and the filtrate was evaporated to dryness in vacuum. The obtained residue was dissolved in ether (400 mL) and washed with water (200 mL) and brine. After drying over magnesium sulfate, the solution was evaporated to give (dibenzylidene amino)-acetonitrile (47.89 g).
[0222] Under nitrogen, an aqueous solution (91mL) of sodium hydroxide (91g, 2.275mol) in a 2L flask was cooled on ice, and then benzyltriethylammonium chloride (2.0g, 0.0088mol, Aldrich) and (dib...
Embodiment 2
[0224] Synthesis Example 2: Scheme 2
[0225]
[0226] Scheme 2, Step 1: Synthesis of methyl (S)-2-(benzyloxycarbonylamino)-4-chloro-4-oxobutanoate
[0227]
[0228] See Synth. Comm. 1993, 23(18):2511-2526. Dissolve N-benzyloxycarbonyl-L-aspartic acid 2-methyl ester (5g, 17.7mmol) in 30ml anhydrous THF, and add 2 Stir at 0°C. At 0°C, thionyl chloride (10.5 g, 88.5 mmol, 5 equivalents) was added to the solution with a syringe, and the solution was refluxed for one hour. The solvent was removed under vacuum and the product was crystallized from dichloromethane / hexane to give methyl 2(S)-2-benzyloxycarbonylamino-3-chlorocarbonylpropionate.
[0229] 1 H NMR(400MHz, CDCl 3 )δ3.48(dd, 1H, J=18.5Hz, J=3.7Hz), 3.56(dd, 1H, J=18.5Hz, J=3.7Hz), 3.74(s, 3H), 4.58(m, 1H) , 5.10 (s, 2H), 5.72 (d, 1H), 7.30-7.35 (m, 5H) ppm.
[0230] Scheme 2, Step 2: (S)-2-(Benzyloxycarbonylamino)-4-oxo-5-phenylpentanoic acid methyl ester
[0231]
[0232] A solution of lithium bromide (2.2g, 25.44mmol, 2.4 equi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com