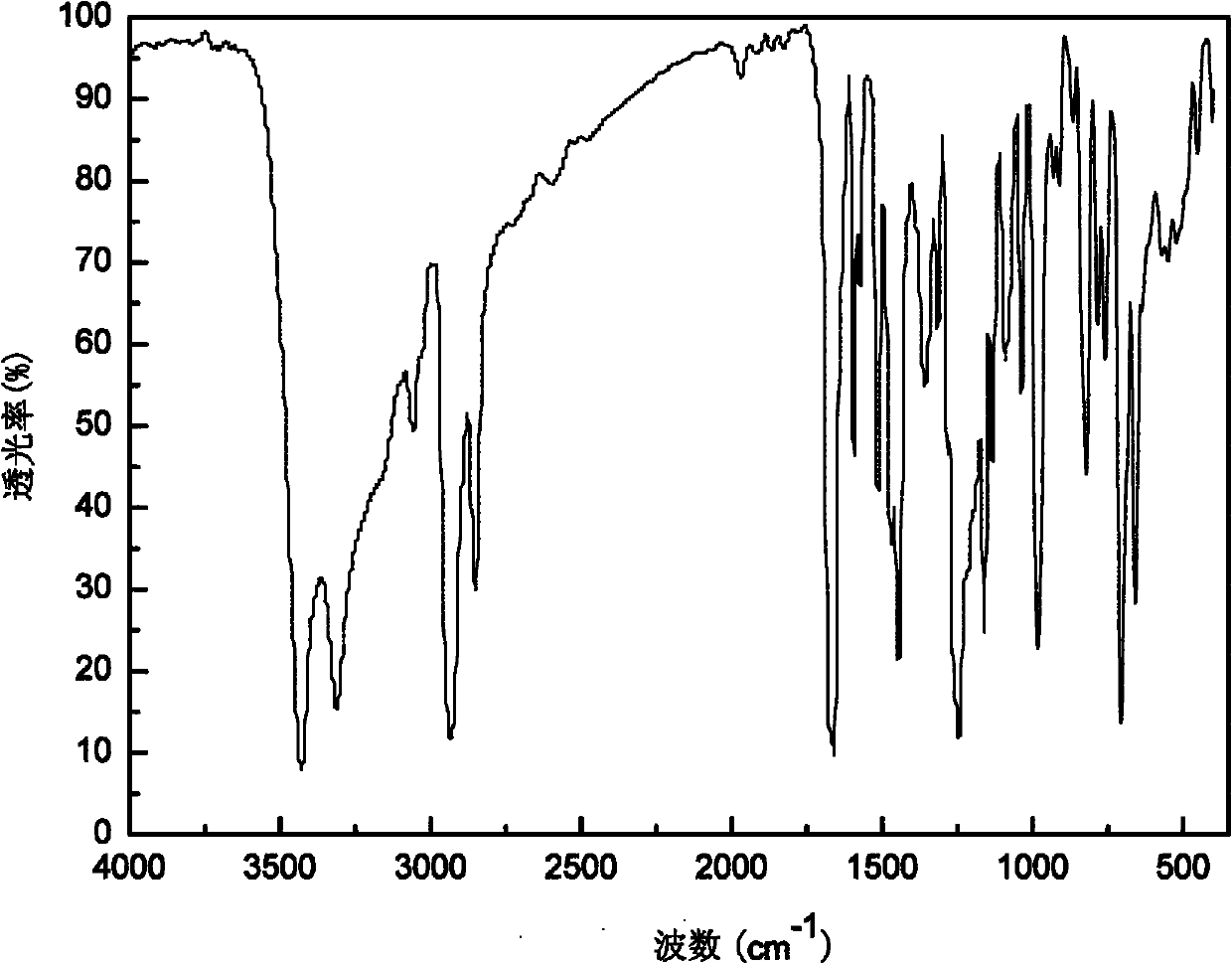
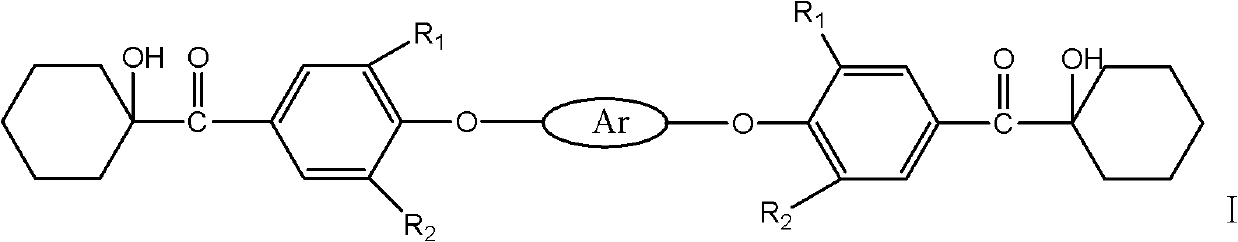
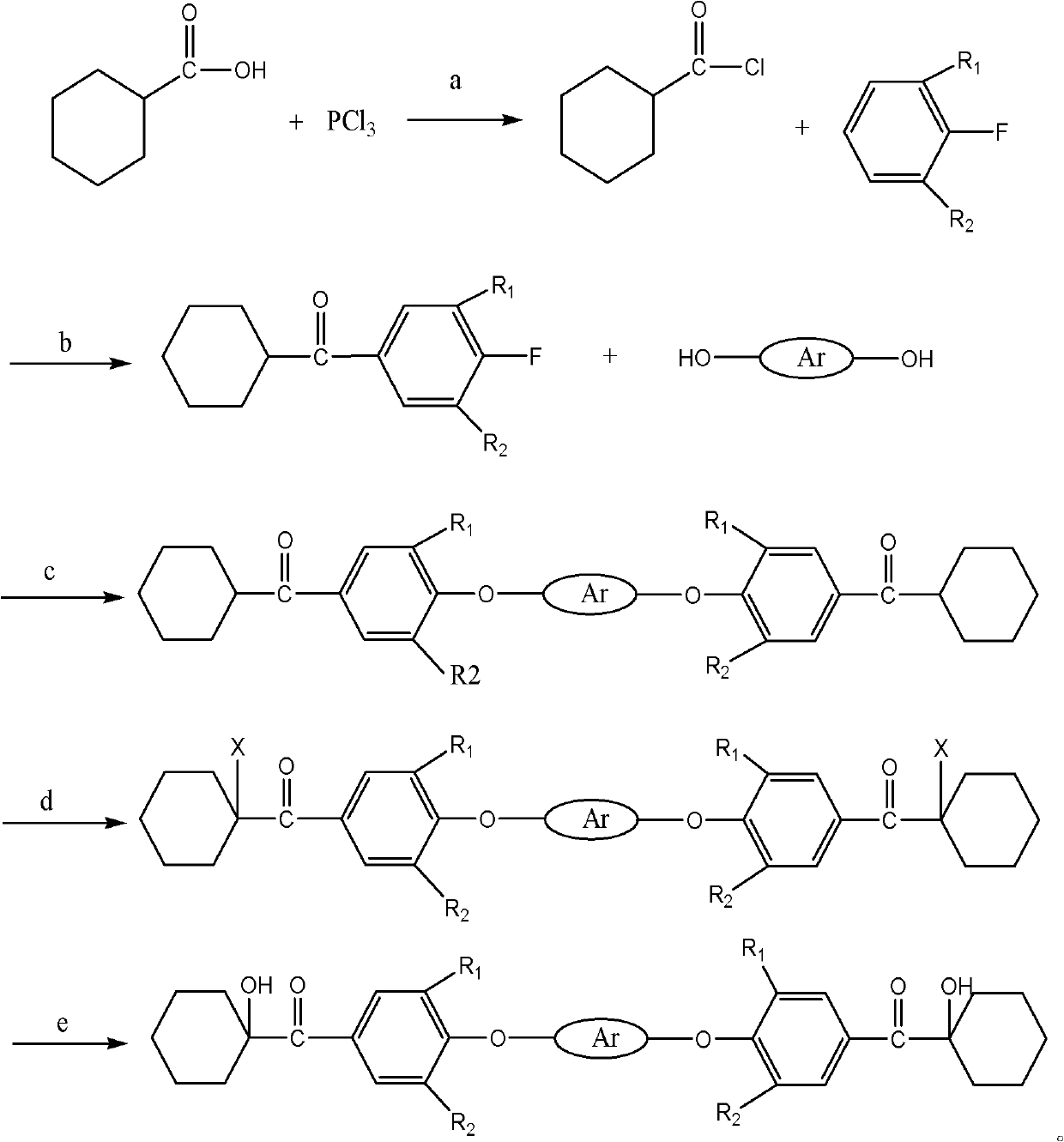
Macromolecule difunctional group alpha-hydroxy-ketone photoinitiator and preparation method thereof
A bifunctional, photoinitiator technology, applied in the field of macromolecular bifunctional α-hydroxyketone photoinitiator and its preparation, can solve the problems of restricted application, easy migration and volatilization of fragments, poor polymer compatibility, etc. The effect of high product yield, good compatibility and easy availability of raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] Add 64g (0.5mol) of cyclohexanecarboxylic acid into a 500mL three-necked flask equipped with stirring, start stirring, and slowly add 30.3g (0.22mol) of phosphorus trichloride dropwise at a controlled temperature of 40°C-50°C, and drop it over in 2 hours , after the dropwise addition was completed, the reaction was continued for 4 hours, left to stand for stratification, and the residue in the lower layer was separated to obtain 70.9 g of cyclohexanoyl chloride, with a yield of 96.8%.
[0032] Take 72g (0.54mol) of anhydrous aluminum trichloride and place it in a reaction flask, add 139.4g (1.45mol) of fluorobenzene, stir, cool to 10°C and slowly add 70.9g (0.48mol) of cyclohexanecarbonyl chloride dropwise, add After completion, raise the temperature to 35°C-40°C and stir for 2 hours. After the reaction, slowly pour the reaction liquid into 10% hydrochloric acid solution, stir vigorously, separate the organic phase, wash, and recover the fluorobenzene by distillation, th...
Embodiment 2
[0037] Catechol was used instead of hydroquinone in Example 1 to obtain 46.5 g of intermediate 1,2-bis[4-cyclohexylmethanone)phenoxy]benzene with a yield of 44.2%.
Embodiment 3
[0039] Resorcinol was used instead of hydroquinone in Example 1 to obtain 60.2 g of intermediate m-1,3-bis[(4-cyclohexylketone)phenoxy]benzene with a yield of 57.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com