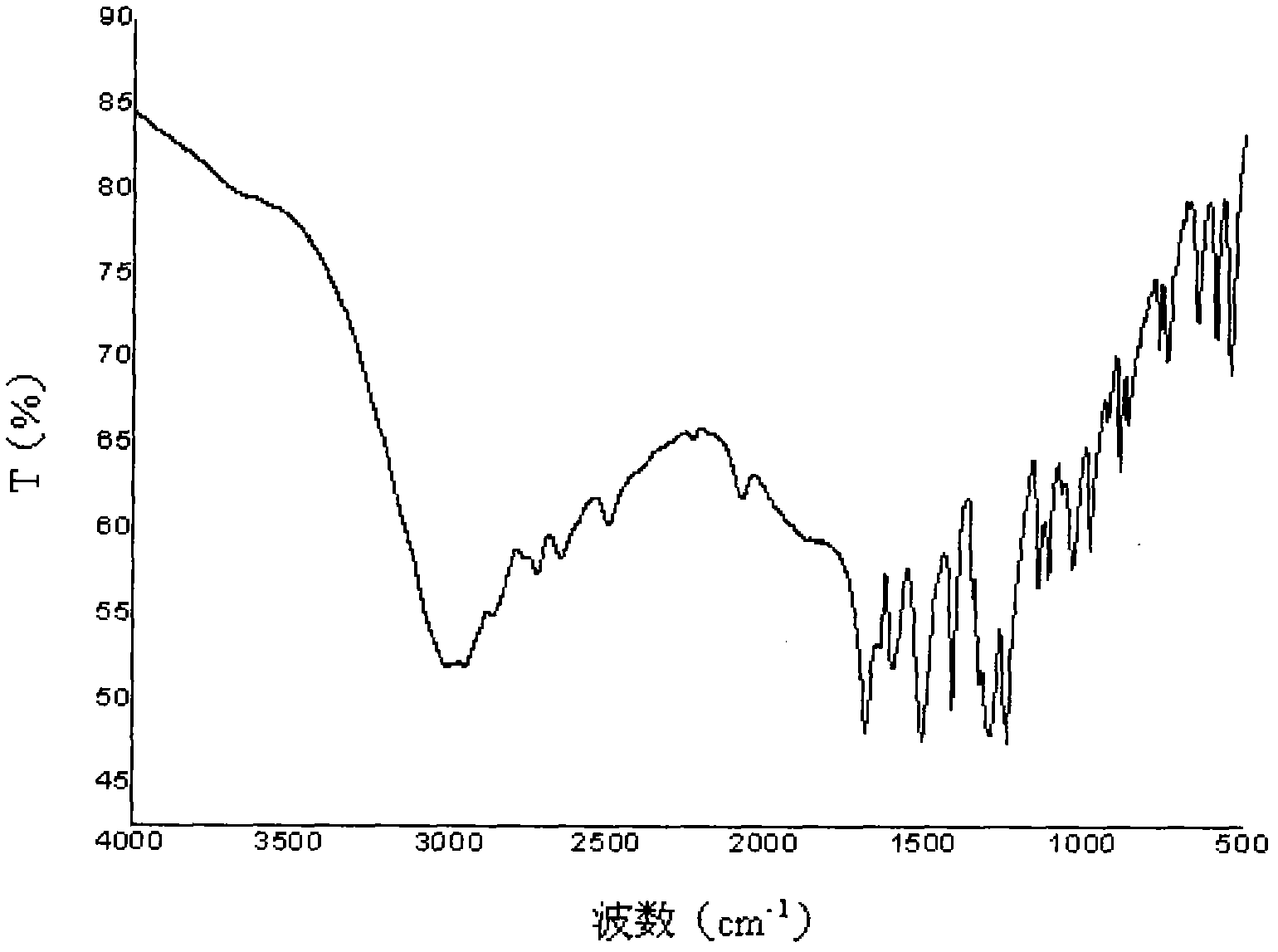
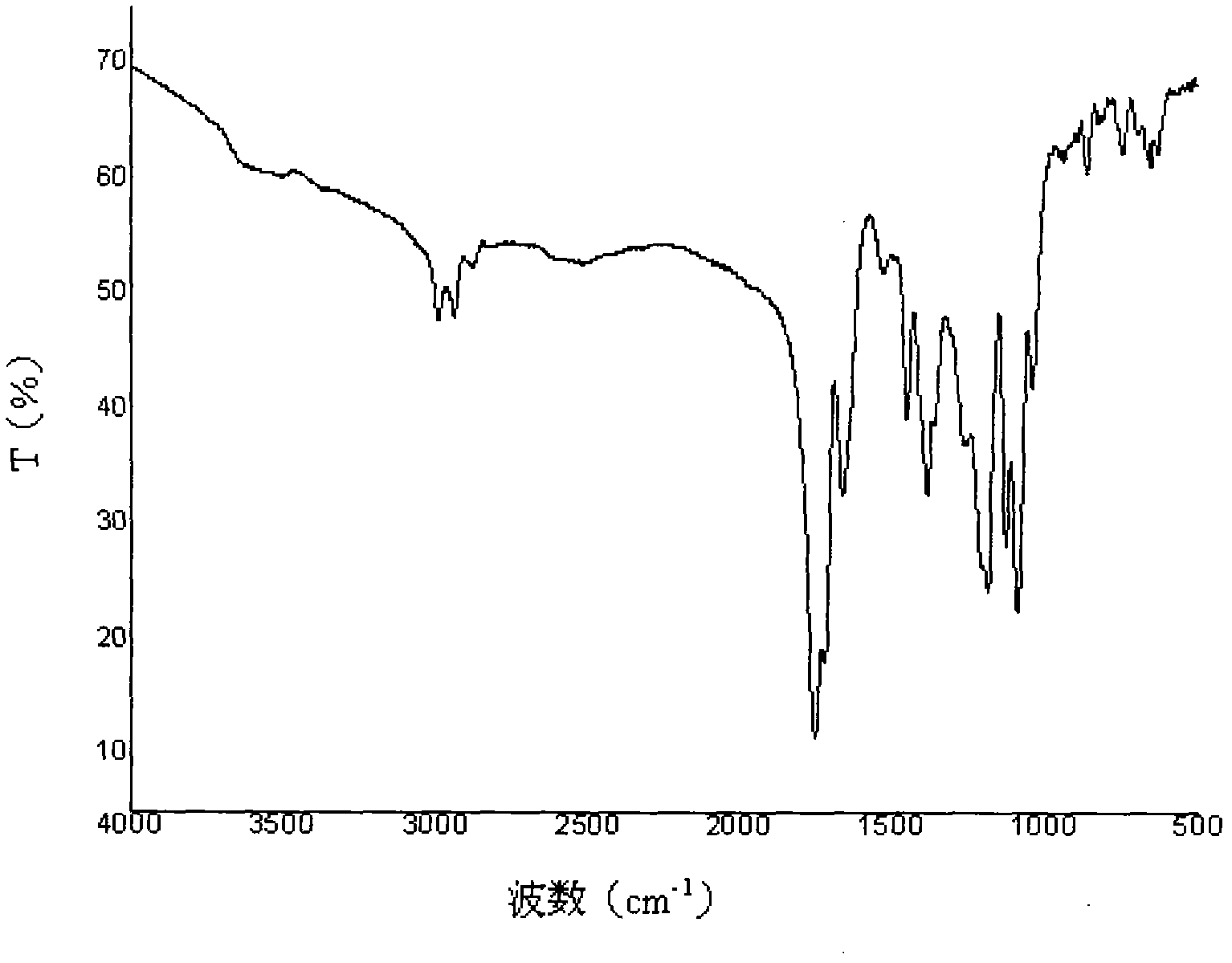
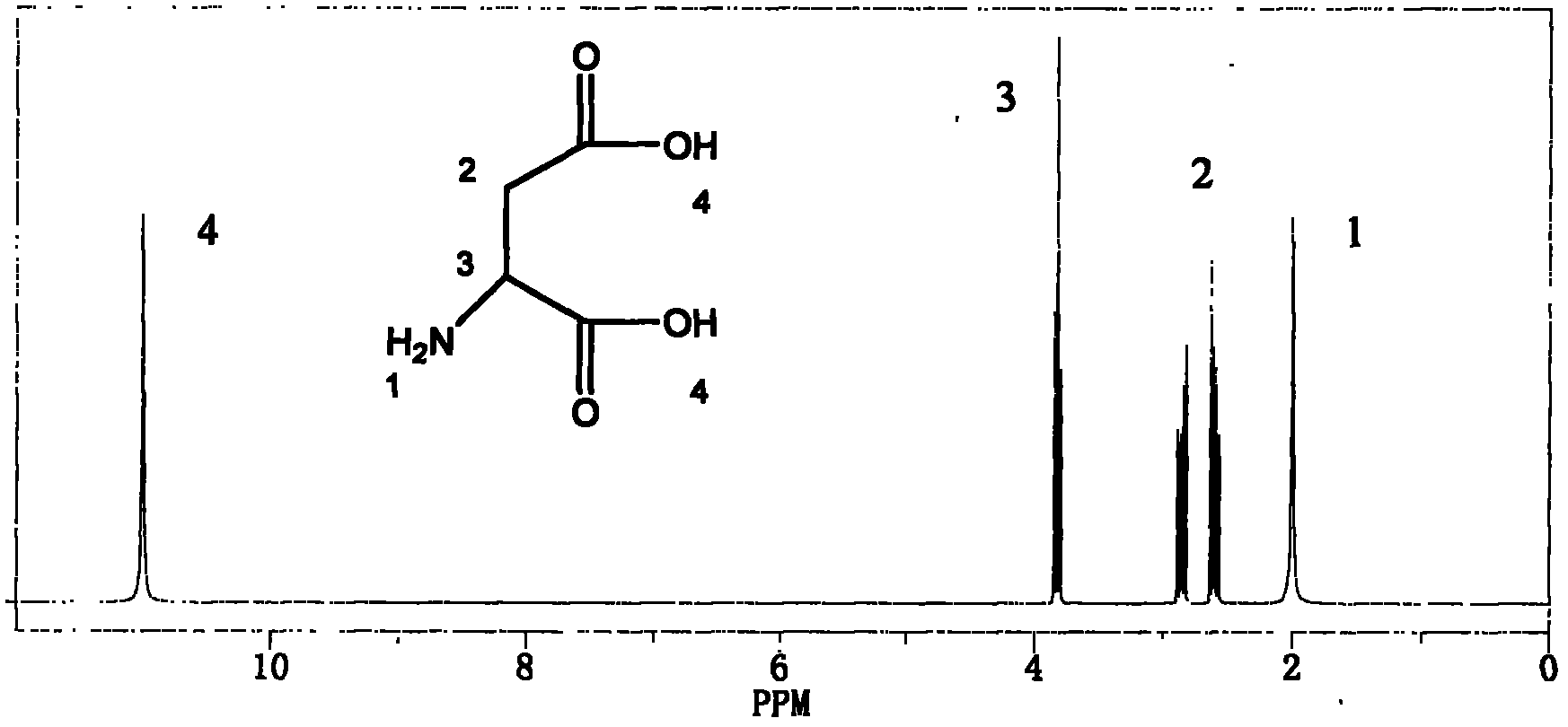
Preparation methods of poly (aspartic acid-co-lactic acid) graft polymer and nanoparticles of poly (aspartic acid-co-lactic acid) graft polymer
A technology of grafting polymer and aspartic acid, applied in nanotechnology, drug combination, pharmaceutical formulation and other directions, can solve problems such as poor hydrophilicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0030] According to the preparation method provided by the present invention, the reaction is carried out under nitrogen, and the conditions of the reaction include: the molar ratio of the aspartic acid to the lactide is 1:1-10, relative to 10 g of light brown solid, the amount of the first organic solvent is 5-20mL, the reaction temperature is 160-180°C, and the contact time is 16-26 hours.
[0031] Since aspartic acid can self-polymerize to form polyaspartic acid at high temperature, lactide can directly react with -OH on polyaspartic acid. Therefore, in the present invention, direct reaction of lactide and aspartic acid is selected to obtain the poly(aspartic acid-co-lactic acid) graft polymer represented by formula (1). Both lactide and aspartic acid used in the present invention are commercially available.
[0032] Preferably, the preparation method provided by the present invention also includes removing N,N-dimethylformamide from the product obtained after the reaction...
Embodiment 1
[0043] Synthesis of Poly(Aspartic Acid-co-Lactic Acid) Grafted Polymer
[0044] Add L-aspartic acid (3.33g, 0.025mol) (Alfar Aesar company, 98%, analytically pure), L-lactide (7.2g, 0.05mol) (Alfar Aesar company, 97%, analytically pure) Into a 50mL single-necked round bottom flask, evacuate for 1 hour to remove oxygen, pass through nitrogen, and under the protection of nitrogen, stir the reaction in an oil bath at 180°C. The solution turned into a yellow transparent liquid. After reacting for 2.5 hours, the temperature was lowered to 160° C. for 21 hours. The reaction solution is viscous light brown liquid. Remove from the oil bath and cool to give a light brown solid which is dissolved in 15 mL of DMF and unreacted lactide is filtered. The filtrate was precipitated in 250 mL of deionized water and washed three times with 100 mL of deionized water. Dry in a vacuum oven at 25° C. for 36 hours to obtain 8.4 g of poly(aspartic acid-co-lactic acid) graft polymer.
[0045] Aft...
Embodiment 2
[0054] Synthesis of Poly(Aspartic Acid-co-Lactic Acid) Grafted Polymer
[0055] Add L-aspartic acid (6.66g, 0.05mol) (Alfar Aesar company, 98%, analytically pure), L-lactide (7.2g, 0.05mol) (Alfar Aesar company, 97%, analytically pure) Into a 50mL single-necked round bottom flask, evacuate for 1 hour to remove oxygen, feed nitrogen, and stir the reaction in an oil bath at 180°C under the protection of nitrogen. The solution turned into a yellow transparent liquid. After reacting for 3 hours, the temperature was lowered to 160° C. for 23 hours. The reaction solution is viscous light brown liquid. Remove from the oil bath and cool to give a light brown solid which is dissolved in 20 mL of DMF and unreacted lactide is filtered. The filtrate was precipitated in 250 mL of deionized water, and washed three times with 200 mL of deionized water. Dry in a vacuum oven at 20°C for 24 hours to obtain 11.1 g of poly(aspartic acid-co-lactic acid) graft polymer.
[0056] After detection...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
| Weight average molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com