Microporous spongy film preparation and preparation method thereof
A microporous sponge and sponge-like technology, which is applied in the direction of pharmaceutical formulations, making medicines into special physical or ingestible devices, and sheet-like delivery, can solve problems such as poor hand feeling, and is beneficial to first-aid applications, shortening the Production time, high bioavailability effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] Preparation method of film for drug film preparation:
[0065] (1) 100g pore forming agent is added to 100g HPMC water slurry, and the weight concentration of HPMC water slurry is 25%;
[0066] The components of the pore-forming agent include microcrystalline cellulose with a particle size of 1 μm and ethanol adsorbed thereon, and the parts by weight of the microcrystalline cellulose and the parts by weight of the solvent are:
[0067] Microcrystalline cellulose 100 parts
[0068] 300 parts of ethanol
[0069] (2) Then adopt the method reported in the literature of the research on nitroglycerin membrane (Chinese Journal of Pharmaceutical Industry, 1977, 12 (5)), apply and form a membrane, and obtain a thin film precursor;
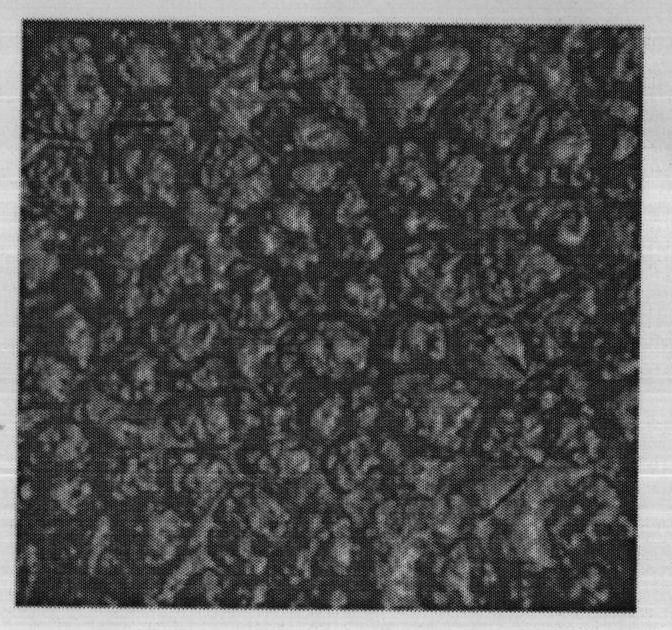
[0070] (3) Dry the thin film precursor obtained in step (2) at 80° C. to obtain the thin film for drug film preparation, with a thickness of 0.06 mm and a micropore diameter of 50 nm. Its electron microscope photo see figure 1 .
[0071] From f...
Embodiment 2
[0073] Preparation method of film for drug film preparation:
[0074] (1) 50g pore forming agent, 1g antioxidant are added to the water slurry of 100g PVA, and the weight concentration of PVA water slurry is 20%;
[0075] The components of the pore-forming agent include chitin with a particle size of 0.5 μm and acetone adsorbed thereon, and the parts by weight of chitin and the parts by weight of the solvent are:
[0076] Chitosan 100 parts
[0077] Acetone 400 parts
[0078] (2) Then adopt the method reported in the literature of the research on nitroglycerin membrane (Chinese Journal of Pharmaceutical Industry, 1977, 12 (5)), apply and form a membrane, and obtain a thin film precursor;
[0079] (3) Dry the thin film precursor obtained in step (2) at 60° C. to obtain the thin film for drug film preparation, with a thickness of 0.1 mm and a micropore diameter of 80 nm. Its electron microscope photo see figure 2 .
Embodiment 3
[0081] Voglibose Microporous Sponge Film:
[0082] Add 100g of pore-forming agent, 2g of voglibose, and 2g of Tween-80 into 100g of HPMC water slurry, and the weight concentration of HPMC water slurry is 25%.
[0083] Others are the same as embodiment 1.
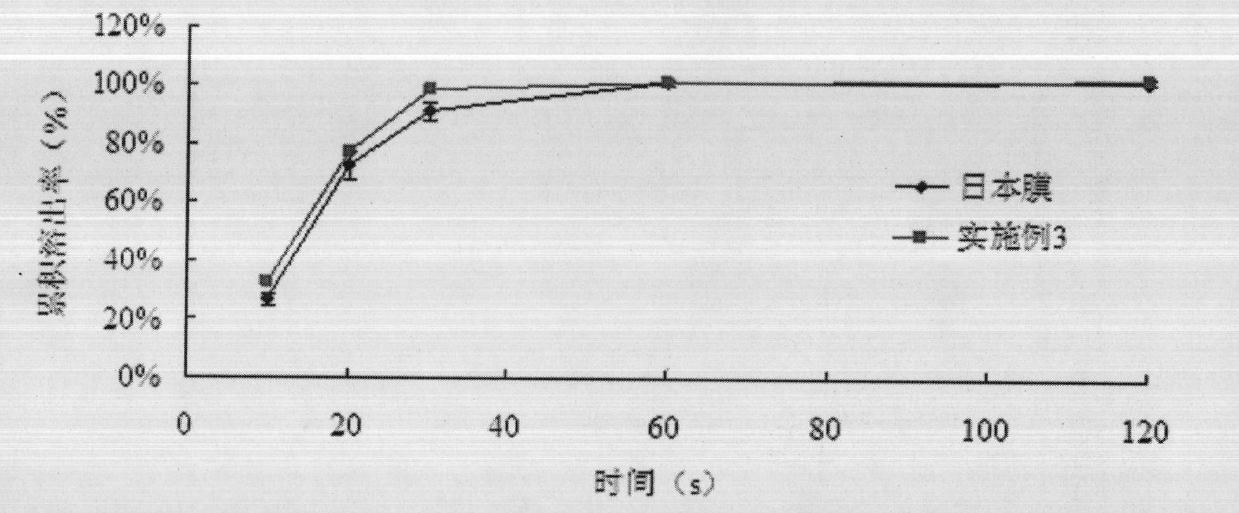
[0084] Get 6 film preparations, clamp them with paper clips respectively, adopt the apparatus for dissolution determination (the third method of appendix XC of the Chinese Pharmacopoeia version in 2010), take phosphate buffer (pH5.8) 100ml as the dissolution medium, and the rotating speed is Minute 30 revolutions, since the test product contacts the dissolution medium, time it immediately, after 10, 20, 30 seconds, 1, 2 minutes respectively, take 1ml of the solution, filter, take the continued filtrate, adopt post-column fluorescence derivatization method, illuminate High performance liquid chromatography (Chinese Pharmacopoeia 2010 edition two appendix VD) is measured and calculated the dissolution amount of each tablet. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Thickness | aaaaa | aaaaa |
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com