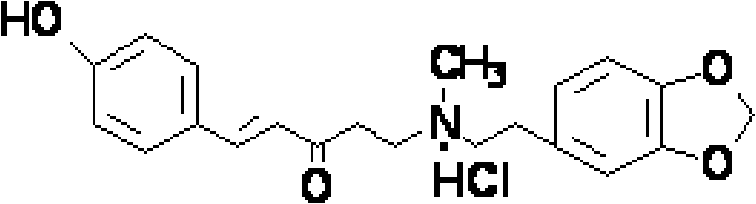
Application of peperphentonamine or salt thereof in preparing drug for preventing/treating encephalopathy
The technology of piperphentonamine salt and piperphentonamine is applied in the field of use of piperphentonamine or its salt in the preparation of medicines for preventing/treating encephalopathy, and can solve the problem that the curative effect of patients with craniocerebral trauma lacks first-level clinical evidence-based Medical evidence and other issues to achieve good therapeutic effect, stable yield, and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] Preventive and therapeutic effects of piperphentonamine and piperphentonamine hydrochloride on cultured brain neuron injury.
[0046] 1. Experimental animals: 18 ordinary Sprague Dawley rats, weighing 250g-280g. Polylysine is a product of Sigma; DMEM medium is a product of Gibco; other reagents are of analytical grade.
[0047] 2. Primary culture of nerve cells
[0048] Pregnant rats were anesthetized with chloral hydrate on day 15, sterilized the chest and abdomen with 75% ethanol, took out the fetal rats under aseptic conditions, peeled off, separated the cortical tissues on both sides, cut them into minces with a scalpel, and transferred them into phosphoric acid containing 0.125% trypsin Digest in the buffer solution for 30min (37°C), discard the digestion solution, add DMED culture solution containing 10% fetal bovine serum, 10% horse serum, 100U / ml penicillin, 100U / ml streptomycin, blow repeatedly with a small-bore pipette Disperse, filter through a 200-mesh cel...
experiment example 2
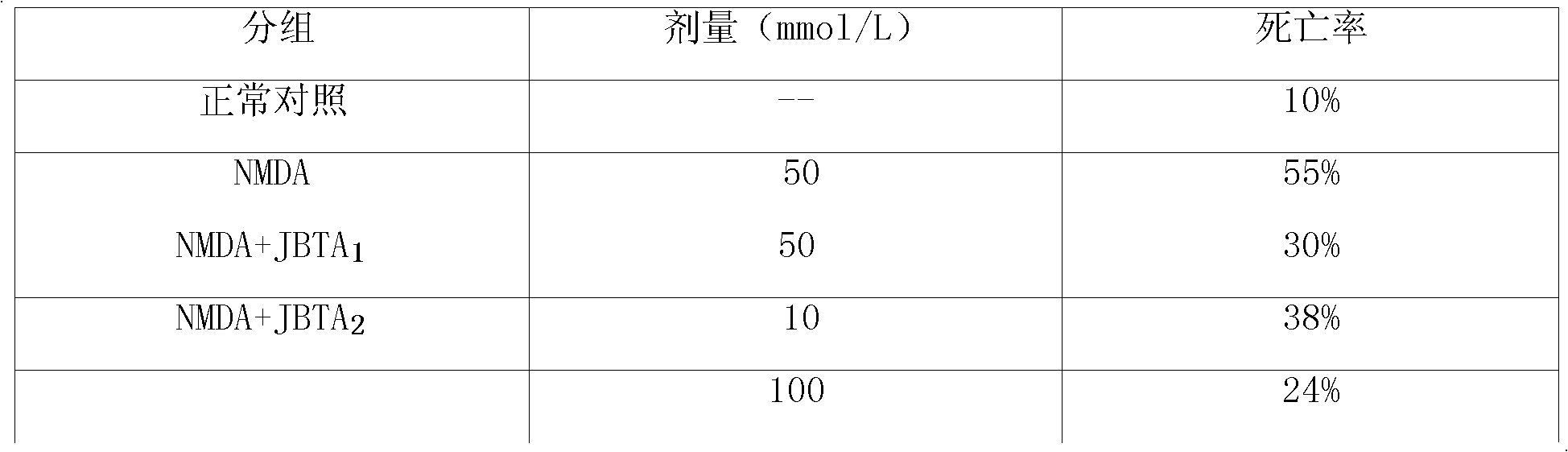
[0060] Therapeutic effects of piperphentonamine and piperphentonamine hydrochloride on mice with blunt traumatic brain injury.
[0061] The automatic recorder for the jumping experiment was from the Instrument and Electricity Department of the Institute of Materia Medica, Chinese Academy of Medical Sciences; the fluorescence spectrophotometer was from HITACHI Corporation of Japan; and the microplate reader.
[0062] Experimental animals: clean grade Kunming mice, weighing 20g-22g.
[0063] Get 70 mice, are randomly divided into normal control group, model group, 2mg / kg piperphentonamine (dilution to appropriate concentration with 5% glucose injection when used, the same below) group, piperphentonamine hydrochloride 1mg / kg group , 2mg / kg group, 4mg / kg group and 4mg / kgATP group, 10 rats in each group.
[0064] After fasting for 16 hours, a mouse model of closed traumatic brain injury was established. Each group was administered intravenously. After 24 hours, the brains of the m...
Embodiment 3
[0070] Neuroprotective therapeutic effect of piperphentonamine hydrochloride in mice with vascular dementia.
[0071] Piperphentonamine hydrochloride: Dilute to an appropriate concentration with 5% glucose injection when used. The automatic recorder for the jumping experiment is a product of the Instrument and Electricity Laboratory of the Institute of Materia Medica, Chinese Academy of Medical Sciences; the fluorescence spectrophotometer is a product of HITACHI Japan; the microplate reader.
[0072] Experimental animals: clean grade Kunming mice, weighing 20g-22g.
[0073] 1. Improvement effect of piperphentonamine hydrochloride on learning and memory dysfunction in cerebral ischemia-reperfusion mice
[0074] Animals were randomly divided into sham operation group, model group, piperphentonamine hydrochloride 1mg / kg group, 2mg / kg group, 4mg / kg group, 10 in each group.
[0075] After fasting for 16 hours, the mouse model of dementia caused by ischemia-reperfusion was establi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com